Veeva provides various standard connections to support and automate business processes between separate Vaults. For example, organizations using both a Veeva QMS and a Veeva Safety Vault can use the Quality-Safety Connection for product quality complaint and adverse event intake. In many cases, a standard Veeva Connection provides a sufficient starting point for an organization’s data transfer needs, enabling quicker configuration and use.
Note: Veeva Connections are a highly complex feature set that can be used in many Vault applications. We strongly recommend working with your Veeva representative or Veeva Services to configure your organization’s specific requirements. Learn more about standard connections at the Veeva Connections Resource Hub, or in a specific connection’s Veeva Connect community.
Standard Connection Features & Benefits
Organizations with multiple Vaults on the same domain can consider a standard Veeva Connection to:
- Reduce workflow steps across organizations by sharing data and documents in real-time with the right teams.
- Increase data quality and traceability back to a Vault which provides the source of truth.
- Simplify an organization’s IT ecosystem by eliminating development and maintenance of custom integrations.
Each connection includes one or more Integrations, or features supporting a given business use case.
Connection Naming & Data Flow
Standard Veeva Connections transfer data between two Vaults within separate application suites, as indicated by the connection’s name. For example, the Quality-RIM Connection sends and receives data between Vaults within the Quality and RIM (Regulatory) application families. This naming convention does not indicate the direction in which data flows, nor does it indicate the required applications within those application suites.
This applies even when:
- A connection is implemented with only one integration, then additional integrations are added later.
- A connection (or a specific integration within that connection) supports only some applications within that family. For example, to use the Quality-RIM Connection’s Enhanced Change Control integration, the Quality Vault must have the QMS application enabled, and the RIM Vault must have Registrations. In contrast, this connection’s Product Transfer integration is supported in any Quality and RIM Vaults.
- A connection’s Integrations and related Integration Rules facilitate a one-way, unidirectional data transfer. All connections are considered to be two-way, or bidirectional, regardless of individual integration behavior.
Note: Your Vault’s Connection and related records in Admin > Connections may be labeled and/or named with “to”. This reflects a legacy naming convention and does not indicate directionality of data flow, nor does it indicate the only supported application. For example, the QMS-RIM Connection record is labeled and named QMS to RIM (qms_to_rim__v), but facilitates bidirectional data flow between RIM applications and Quality applications (QMS, QualityDocs).
Clinical Data Applications & Connections
Veeva EDC and Veeva eCOA are Clinical Data suite applications, and their connections with the Clinical Operations and Safety suites are described on this page.
For information about these applications and other Clinical Data connections not covered here, visit Veeva Clinical Data Help and the Veeva Connections Resource Hub.
Vault Domains
Standard Veeva Connections require that connected Vaults share the same domain. See additional details about Vault domains.
Standard Integrations
Each Veeva Connection is supported by a set of individual integrations, or features which support a specific business use case.
While the functionality of an integration is unique to the connection, some common integrations include:
- Adverse Event Intake
- Document Exchange
- Product Data
- Product Quality Complaint Intake
- Study Data
A given connection’s integration functionality is determined by its Integration Points and supporting Integration Rules.
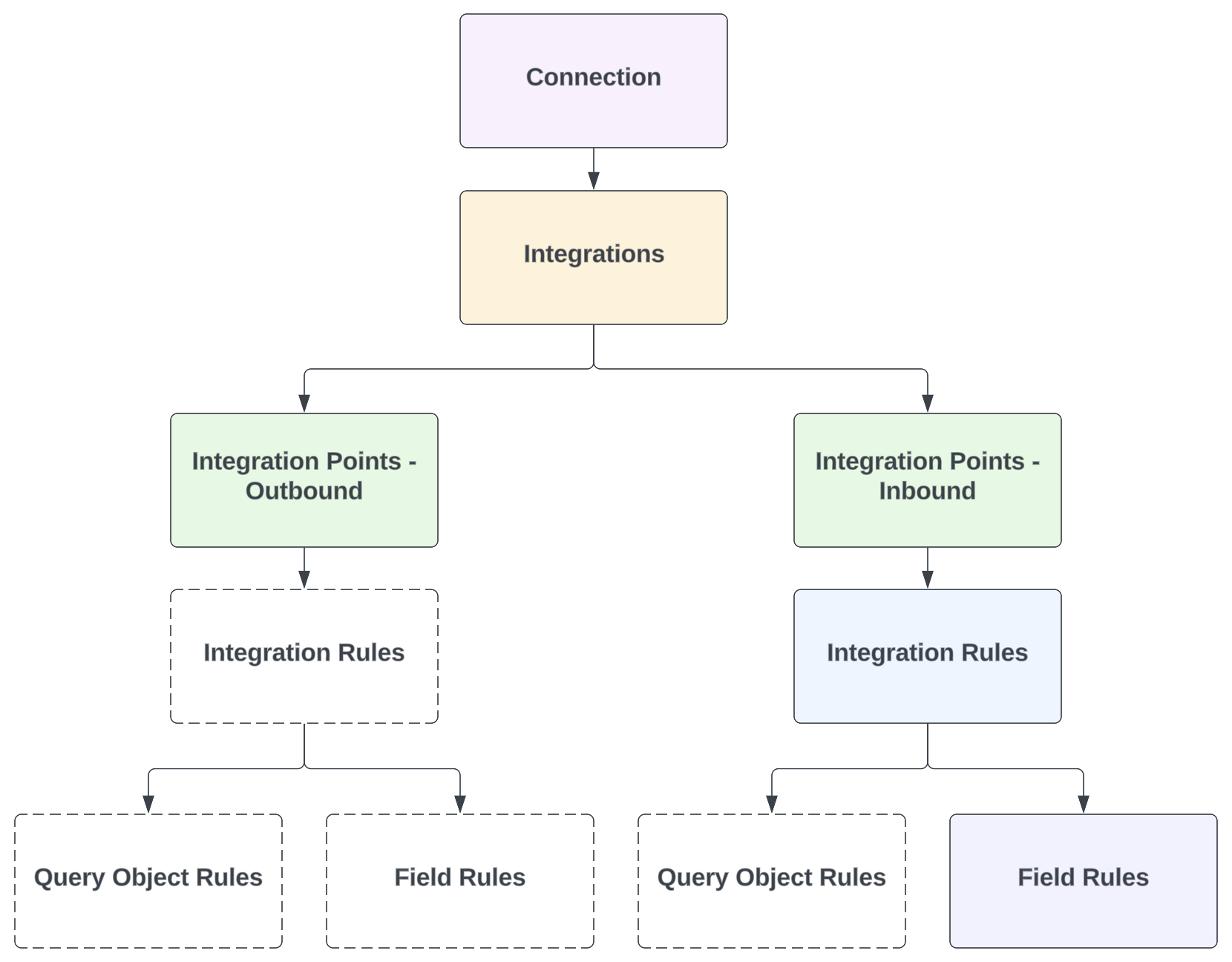
Connection & Integration Structure
Veeva Connections are represented in each connected Vault by several record types. These records can be found in Admin > Connections.
Each Vault includes one Connection record for the connection itself. Then, each integration (feature) is represented by an Integration record, which has one or more related Integration Points, Integration Rules, Query Object Rules, and/or Field Rules.
Depending on the integration, some standard (Veeva-provided) records may not be included. For example, in a Quality Vault, the Quality-RIM Connection’s Document Exchange integration currently includes only one Integration Point (inbound). Then, this single Integration Rule has Field Rules only. A high-level overview of these relationships is shown in the diagram below.
Integration Dependencies
Some connections require one integration to be implemented prior to or in parallel with another integration. For example, the Quality-RIM Connection’s Product Transfer integration must be in use along with the Enhanced Change Control integration. Similarly, the Enhanced Change Control integration supersedes the Variation Management integration, meaning organizations that have not yet implemented Variation Management should use Enhanced Change Control instead.
Similarly, a given integration may require that an application-specific feature is configured in that Vault. For example, the Quality-RIM Connection’s Enhanced Change Control integration additionally requires that the Quality Vault (QMS) is configured for Change Control.
Where required, a connection’s individual listing on this page notes any integration dependencies.
Clinical Operations Connections
Clinical Operations-EDC
The table below summarizes the integrations (features) supported for the Clinical Operations-EDC Connection.
| Integration | Required Applications |
|---|---|
| Enhanced Monitoring | CTMS, EDC |
| Final CRF | eTMF, EDC |
| Protocol Deviation Management | CTMS, EDC |
| Site Payments | Payments, EDC |
| Study Data Transfer | CTMS, EDC |
| Subject Tracking | CTMS, EDC |
See additional details about configuring this connection.
eCOA-Clinical Operations
The table below summarizes the integrations (features) supported for the eCOA-Clinical Operations Connection.
| Integration | Required Applications |
|---|---|
| Study Data Transfer | Any Clinical Operations app, eCOA |
| End of Study Media | eTMF, eCOA |
See additional details about configuring this connection.
Quality-Clinical Operations
The table below summarizes the integrations (features) supported for the Quality-Clinical Operations Connection.
| Integration | Required Applications |
|---|---|
| Issue Management | QMS with Deviations configured, CTMS |
| Study Data Transfer | Any |
See additional details about configuring this connection.
RIM-Clinical Operations
The table below summarizes the integrations (features) supported for the RIM-Clinical Operations Connection.
| Integration | Required Applications |
|---|---|
| Document Exchange | eTMF, Submissions |
| Product Data Transfer | Any |
| Study Data Transfer | Any |
| Submission Tracking | Study Startup, Submissions |
See additional details about configuring this connection.
Safety-Clinical Operations
The table below summarizes the integrations (features) supported for the Safety-Clinical Operations Connection.
| Integration | Required Applications |
|---|---|
| Safety Letters | Safety, eTMF for Document Exchange. Site Connect is required to send a Safety Distribution to sites. |
| Study Data Transfer | Any |
See additional details about configuring this connection.
Study Training-Clinical Operations
The Study Training-Clinical Operations Connection forms the basis of the Study Training application. As such, the table below lists the individual integrations (features) which support the Study Training application overall.
| Integration | Required Applications |
|---|---|
| Study Data Transfer | N/A |
| Study Persons Transfer | N/A |
| Document Exchange | N/A |
| User Transfer | N/A |
See additional details about setting up Study Training.
Regulatory (RIM) Connections
RIM-Clinical Operations
See Clinical Operations Connections.
RIM-PromoMats
The table below summarizes the integrations (features) supported for the RIM-PromoMats Connection.
| Integration | Required Applications |
|---|---|
| AdPromo Submission Management | Submissions, PromoMats |
| Document Exchange | Submissions, PromoMats |
| Product Data Transfer | Any |
See additional details about configuring this connection.
Quality-RIM
The table below summarizes the integrations (features) supported for the Quality-RIM Connection.
| Integration | Required Applications | Dependencies |
|---|---|---|
| Document Exchange | QualityDocs, Submissions | None |
| Enhanced Change Control | QMS with Change Control, Registrations | - Requires Product Transfer - Supersedes Variation Management |
| Product Transfer | Any | Required for Enhanced Change Control |
| Variation Management | QMS with Change Control, Registrations | Superseded by Enhanced Change Control |
See additional details about configuring this connection.
Safety-RIM
The Safety-RIM Connection supports Product Data Transfer between any Safety and RIM Vault.
See additional details about configuring this connection.
Safety Connections
Safety-Clinical Operations
See Clinical Operations Connections.
Safety-EDC
The Safety-EDC Connection supports Adverse Event Intake between any Safety and EDC Vault.
See additional details about configuring this connection.
Safety-RIM
See Regulatory (RIM) Connections.
Medical-Safety
The Medical-Safety Connection supports Adverse Event Intake between any Safety and Medical Inquiry Vault.
See additional details about configuring this connection.
Quality-Safety
The table below summarizes the integrations (features) supported for the Quality-Safety Connection.
| Integration | Required Applications |
|---|---|
| Adverse Event Intake | Safety, QMS with Complaints |
| Product Quality Complaint Intake | Safety, QMS with Complaints |
See additional details about configuring this connection.
Quality Connections
Medical-Quality
Quality-Clinical Operations
See Clinical Operations Connections
Quality-LIMS
The Quality-LIMS Connection forms the basis of the LIMS application. As such, the table below lists the individual integrations (features) which support the LIMS application overall.
| Integration | Required Applications |
|---|---|
| Lab Investigation Transfer | N/A |
| Reference Object Transfer | N/A |
| Document Exchange | N/A |
See additional details about configuring this connection.
Quality-RIM
See Regulatory (RIM) Connections.
Quality-Safety
See Safety Connections.
Study Training-Clinical Operations
See Clinical Operations Connections.
Commercial Connections
Medical-CRM
The Medical-CRM Connection supports Case Intake between any Vault CRM and Medical Inquiry Vault.
See additional details about configuring this connection.
Medical-Quality
The Medical-Quality Connection supports Product Quality Complaint Intake between any Medical Inquiry Vault and a QMS Vault with Complaints.
See additional details about configuring this connection.
Medical-Safety
See Safety Connections.
PromoMats-CRM
Organizations using both a Veeva PromoMats Vault and Vault CRM can use the standard PromoMats-CRM Connection.
See additional details about configuring this connection.
PromoMats-Medical
The PromoMats-Medical Connection supports Document Exchange between any Medical Inquiry Vault and a PromoMats Vault.
See additional details about configuring this connection.