Using the Safety-EDC Connection, you can exchange data in near-real time between Safety and Clinical Data EDC Vaults. To use the Safety-EDC Connection, you must configure the relevant components, connect any existing data, and activate the connection. This setup requires configuration steps in both Vaults.
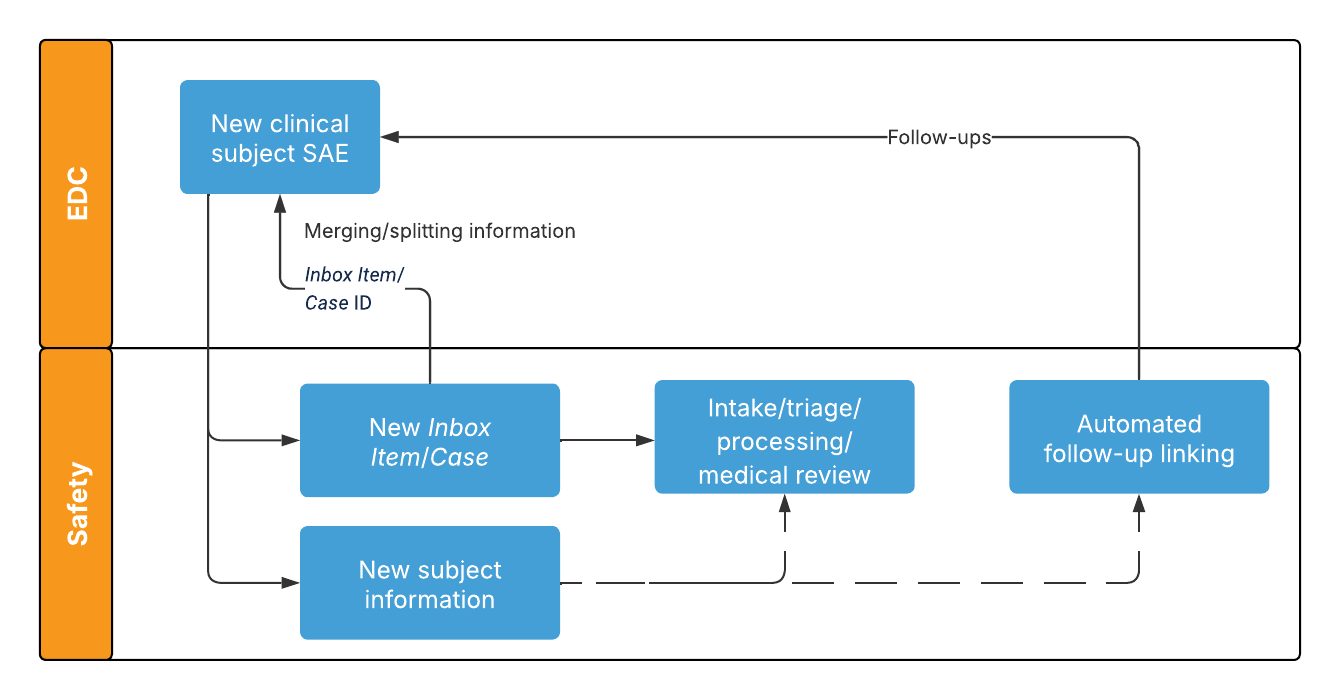
The following diagram outlines how the Safety-EDC Connection exchanges data for new clinical subject SAEs:
For information on how the form maps to Safety Vaults, see Configuring Safety-EDC Connection Studies.
Configuration Overview
You must complete the following steps in your Safety and EDC Vaults to configure the standard connection:
- Review the connection components and adjust as needed to fit your organization’s business processes.
- Configure your Vaults.
- Establish the Connection between your Safety and EDC Vaults.
Safety-EDC Connection Components
The components in the following sections support the standard Safety-EDC Connection.
Connections
The following Connection records are available from Admin > Connections:
- Safety-EDC Connection (
safety_cdms_connection__v) is available in your Safety Vault. - Safety-EDC Connection (
safety_cdms_connection__v) is available in your EDC Vault.
Integrations & Integration Points
The Integration and Integration Point objects enable Vault to manage the message queues and define what documents and data the connection maps from one (1) Vault to another. These records are read-only and are used by Vault to manage the connection and track any connection errors.
| Integration Name | Integration Point Name(s) | Sent From | Sent To | Included Data |
|---|---|---|---|---|
| Safety-EDC: Serious Adverse Events | Safety-EDC: Safety Case Outbound | EDC | Safety | SAE and Subject information |
| Safety-EDC: Serious Adverse Events | Safety-EDC: CDMS Safety Case Inbound | Safety | EDC | SAE and Subject information |
| Safety-EDC: Serious Adverse Events | Safety-EDC: Case Information | EDC | Safety | SAE Case information |
| Safety-EDC: Serious Adverse Events | Safety-EDC: Case Information | Safety | EDC | SAE Case information |
| Safety-EDC: Serious Adverse Events | Safety-EDC: Safety Inbox and Case IDs | Safety | EDC | Inbox IDs and Case IDs |
Queues
This feature includes four (4) standard message processing queues: two (2) in the Safety Vault and two (2) in the EDC Vault. You can access and manage these queues from Admin > Connections > Spark Queues.
In the Safety Vault:
- Safety-EDC SAE Inbound (
cdms_to_safety_sae_inbound_queue__v) - Safety Outbound Messaging Queue (
cdms_to_safety_sae_outbound_queue__v)
In the EDC Vault:
- Safety-EDC SAE Outbound (
cdms_to_safety_sae_outbound_queue__v) - Safety-EDC SAE Inbound (
cdms_to_safety_sae_inbound_queue__v)
User Exception Objects
Vault includes the User Exception Message object and its child object, User Exception Item, to help you track and resolve any errors that occur with your Safety-EDC Connection. If your Safety Vault integration can’t process an incoming message, Vault creates a User Exception Message record to capture the failure. Vault also creates individual User Exception Item records for each item that failed on the related Integration Point record in the outbound Vault.
You can view and manage these messages from Admin > Connections > User Exception Messages. If you want specific users or groups to receive notifications when Vault creates User Exception Message records, you must create a custom lifecycle for the User Exception Message object and configure a notification entry action.
A User Exception Message means that you need to update your configuration. After you update the configuration, Vault attempts to resolve any errors the next time the connection runs. You can also select the Reprocess Request action to run the connection and resolve errors.
Configuring Your Vaults
To configure your Safety and EDC Vaults to support the Safety-EDC Connection:
- Follow the steps in Veeva Clinical Data Help.
- Configure your Safety Vault.
- Configure Study and Study Product matching.
Configuring Your Safety Vault
After completing all the required configurations in Veeva Clinical Data Help, complete the following in your Safety Vault:
- Enable the Safety-EDC Connection.
- Optional: Configure custom field mappings to populate mapped fields with data from the EDC Vault on the corresponding subject information and case objects in your Safety Vault.
- Create the appropriate sponsor Organization to which you will assign the applicable Studies, Products, and Study Products. This record must be a Sponsor type of organization.
- Configure Case Access Group Security to control user access to subject information on SAE data received from EDC Vaults.
- For the applicable Case Access Groups, create a Case Assignment Rule with an Intake Method for the Safety-EDC Connection.
- For the applicable Case Access Group Assignments, update the Subject Information Review field to Subject Information Review.
- Optional: Enable Automated Classification of Inbox Item Significance and configure Significance Criteria rules to assign Significance values to Inbox Items generated by the connection.
- Optional: Configure the Copy to new action on the applicable states of the Connection object lifecycle to connect to multiple EDC Vaults.
- Optional: Configure Merge to In-Flight Case.
- Optional: Configure Automated Case Promotion.
- Configure Intake Settings.
- For the Safety-EDC Connection transmission profile, ensure the Enable Auto Promote field is Yes.
Note: Vault does not support the Promote to Multiple Cases action for Inbox Items generated by the Safety-EDC Connection. We recommend that you configure the action to perform conditionally so that it cannot run on Inbox Items with an Intake Method value of Safety-EDC Connection.
Configuring Custom Field Mappings
You can map supported fields in your EDC Vault to your Safety Vault so that the mapped fields automatically populate with the data received from the Safety-EDC Connection on the related subject information and case objects in your Safety Vault. The fields must have the same names on the Safety-related Form Type in EDC as well as on the corresponding objects in your Safety Vault. When searching for mapped fields on case objects in your Safety Vault, Vault first checks for any matching custom (__c) fields. If no custom fields exist, Vault checks for applicable standard fields (__v).
To map custom fields:
- In your Safety Vault:
- Add custom fields to the relevant EDC subject information objects.
- Optional: Add custom fields to the relevant case objects for any fields you want to map that do not already exist as standard fields. Ensure the names have the exact same values as the corresponding fields you added to the EDC subject information objects.
- In your EDC Vault, map the custom fields to the corresponding Form Types. Using the table below, ensure the names have the same name values as the custom fields you added to the corresponding EDC subject information objects in your Safety Vault.
Note: If you previously configured mapping to custom fields on case objects in your Safety Vault and would like to map to the equivalent standard field instead, unmap your custom mapping in your EDC Vault and add the applicable replacement mapping.
| EDC Subject Information Object in Safety | Case Object in Safety | Form Type in EDC |
|---|---|---|
| CDMS Subject Adverse Event | Case Adverse Event | Safety Case Initiation Event |
| CDMS Subject Medical History | Case Medical History | Medical History Form |
| CDMS Subject Drug History | Case Drug History | Drug History Form |
| CDMS Subject Case Product | Case Product | ConMed Form |
| CDMS Subject Case Product Dosage | Case Product Dosage | Study Drug Form |
| CDMS Subject Test Result | Case Test Result | External Labs Form |
| CDMS Subject Cause of Death | Case Cause of Death | In Case of Death Form |
| CDMS Subject Information | Case | Patient Characteristics From |
Note: If these fields are not mapped correctly, users cannot select the related subject in the Add Relevant Subject Information dialog after running the Add Relevant Subject Information action.
Supported Fields
Vault supports the following object field types for mapping custom fields between EDC and Safety Vaults:
- Date
- Number
- Object (for the Controlled Vocabularies object)
- Picklist
- Text
- Yes/No
Sending SAE Reporter Details
You can configure the general settings in your EDC Vault so that the connection sends reporter details to Safety for all SAEs. To support this, ensure the Reporter field value on the Safety Settings page in your EDC Vault is Full Site Information.
Study & Study Product Matching
Vault can automatically match and populate the appropriate study-related fields on records created and updated from data received from the Safety-EDC Connection. To support matching, Study and Product records must have the same names in your Vaults so that Vault can automatically match and populate the appropriate product- and study-related fields on records created and updated from transferred EDC data. To do this:
- Ensure that Studies and Products have the same values in your Safety and EDC Vaults for the following fields to ensure matching:
- Studies must have matching Study Numbers (
name__v). - Products must have matching Product Names (
name__v). - Study Products must have matching Names (
name__v).
- Studies must have matching Study Numbers (
- In your Safety Vault:
- Create the appropriate Study Product Placeholders.
- Add Study Products with the appropriate matching Blinded Names to the applicable Studies.
- Select the appropriate “Sponsor” type of Organization for the Studies, Products, and Study Products.
If your organization also utilizes the Safety-Clinical Operations, Safety-RIM, and Clinical Operations-EDC Connections, Vault matches Studies between all Vaults based on the matching Link (link_sys) values. If there are no common Link values, Vault matches studies based on the Study matching logic.
Establishing the Connection
When you’ve made all required configurations described above, establish the connection between your Safety Vault and EDC Vaults. See Creating & Managing Connections for detailed instructions.
Multiple Connections
You can connect your Safety Vault to multiple EDC Vaults. Use the Copy to new action on an existing connection to deep copy an existing connection, and its related components, in the same status as the copied components.
To copy an existing connection:
- Navigate to Admin > Connections.
- From the All Actions menu of the connection you want to copy, select Copy to new. This action is executable only for Safety-EDC Connections.
- Optional: Change the label of the newly generated Connection.
Note: Vault supports up to ten (10) EDC connections in a Safety Vault. Contact your Veeva Representative if you require additional connections.