Creating an Event record is the recommended first step for many regulatory processes, as the records containing details pertaining to the event itself (such as the impacted product and its product variants, plus various types of packaging and their shelf life details) serve as the initial entry point for regulatory data that is eventually used across similar records for impacted markets via regulatory objectives and submissions.
The Create & Manage Event Details wizard provides a guided user interface for creating, updating, replacing, and withdrawing details in a few steps: Users can launch the wizard from an Event record, select one or more applicable changes (for example, adding new Drug Packaging details and withdrawing another), populate the required information, then review and confirm the results in a simplified view.
Along with the Create Related Records wizard for managing these details later in the process across impacted markets, bulk Event detail management greatly reduces repetitive data entry, increasing efficiency and enhancing data integrity.
Note: The Create & Manage Event Details wizard is only available for RIM Registrations.
Configuration Overview
To set up bulk Event detail creation and management in your Vault:
- Configure fields and page layouts for the various included Event, Submission, and Regulatory Objective relationship objects. See Configuring Relationship Objects for more information.
- Configure the Event object and object lifecycle with the supporting object actions and state types. See Configuring the Event Object Lifecycle & Actions for details.
- Update and confirm Admin and user permissions for working with the wizard and its various records.
- Update the Veeva-provided Event Change Details records relevant to your organization, or create your own. See About Event Change Types & Details and Managing Event Change Types & Details for more information.
Supporting Event Change Items & Labeling Concepts
When gathering an event’s details, wizard users may want to be able to include the Event’s related Change Items and, optionally, any Labeling Concepts related to those items.
To enable this capability for Change Items:
- Activate the following Event Change Details and update them according to your organization’s requirements:
- Product Change Details
- Packaging Change Details
- Labeling Concept Details
- Add the Event Change Details you activated in Step 1 to the desired Event Change Types. We recommend that Product Change Details and Packaging Change Details are arranged such that they are the first details listed within the selected type.
- Navigate to Admin > Settings > Application Settings and enable the Exclude change item lifecycle states from Create Event Details setting by selecting the Change Item object Lifecycle States which Vault should filter when selecting items to display in the wizard. Your selections depend on your organization’s requirements, however we recommend selecting the below states.
- Approved, Ready for Implementation
- Cancelled
- Completed
- Inactive
- Not Applicable
- Not Approved
- Partially Approved
- Planned
- Review the Allow creation of change items in Create Event Details Application Setting. This setting is enabled by default but can be disabled at any time, for example to allow the Quality-RIM Vault Connection to create these records instead.
- If your configuration allows users to create Change Items, navigate to Admin > Configuration > RIM Object Configurations and locate the change_item__v configuration.
- Add any custom (
__c) fields as desired, setting the Create Display Order Registrations Setting accordingly. - Review the default RIM Field Configurations and adjust the Create Display Order as needed.
- Add any custom (
If your organization uses Labeling Concepts, review the Origin picklist values and related Labeling Concept object field configuration. To fit your organization’s requirements for Labeling Concepts, you can add any custom values and/or inactivate the provided standard (__v) values. You may also consider requiring the corresponding field within the Labeling Concept object by selecting the User must always enter a value (required) configuration option.
Configuring Relationship Objects
Various Event, Submission, and Regulatory Objective relationship objects require configuration per the below. See Relationship Object Inventory for the full listing of all in-scope objects.
- The wizard references the various impacted relationship objects via the Event Change Type’s Related Change Type field. To configure this, add the Related Change Type picklist field to all Event, Submission, and Regulatory Objective relationship objects, as well as their page layouts. See Relationship Object Inventory for the full listing.
- For Event relationship objects only: For the Name field, select the System manages field value (read-only) configuration option.
Configuring the Event Object Lifecycle & Actions
The Event object lifecycle requires you to configure select Event object actions, then add them to the desired object lifecycle states to allow users to execute them from an Event record. You must also configure an In Review lifecycle state, which allows users to generate a preview of their changes.
Configuring Event Object Actions
Within the Event object, create the following object actions, or ensure they are active:
- Create Event Details
- Manage Event Details
- Review Event Details
- Clear Preview
Configuring the Event Object Lifecycle
To configure your Vault’s Event object lifecycle for bulk detail creation:
- If not already configured for the Create Related Records wizard, create an In Data Review state, then map it to the In Data Review state type.
- Configure user actions for Create Event Details and Manage Event Details in any lifecycle state, except for In Data Review.
- Configure the following user actions in the In Data Review state:
- Review Event Details
- Clear Preview, if not already configured for creating related records.
About Event Change Types & Details
Event Change Type and Event Change Detail configuration records are mandatory for creating and managing Event details in bulk, and they determine whether and how users complete fields in the wizard.
While your organization may require additional Event Change Types or Event Change Details, Veeva provides sample records as a starting point. See Managing Event Change Types & Details for specific instructions on how to update the Veeva-provided records, or create your own.
About Event Change Types
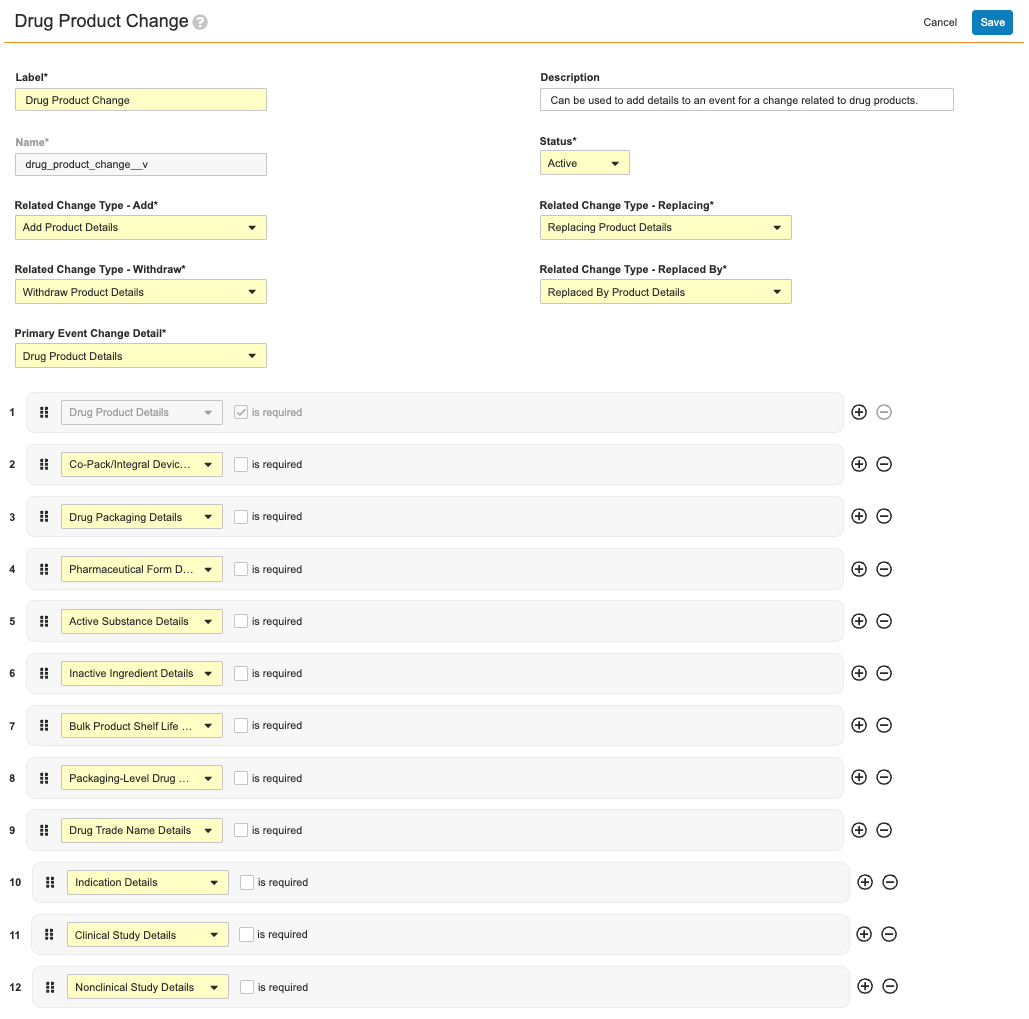
Event Change Types define the required and optional details for a specific kind of change. For example, the Drug Product Change Event Change Type record defines:
- Users’ ability to add, withdraw, and replace details for an event change related to drug products in general, as defined by the Related Change Type fields (Add, Withdraw, Replacing, and Replaced by).
- The primary and additional Event Change Details to be referenced by the wizard during processing. In this example, the Drug Product Details record is populated in this type’s Primary Event Change Detail field, and therefore the defined details must always be collected from users in the wizard. Conversely, the 11 remaining Change Details are not required, and are therefore at users’ discretion when working in the wizard.
About Event Change Details
Event Change Details are the building blocks of Event Change Types: A single Event Change Detail record specifies the Event relationship object (and its object types, where applicable) containing the field data to be collected in the wizard.
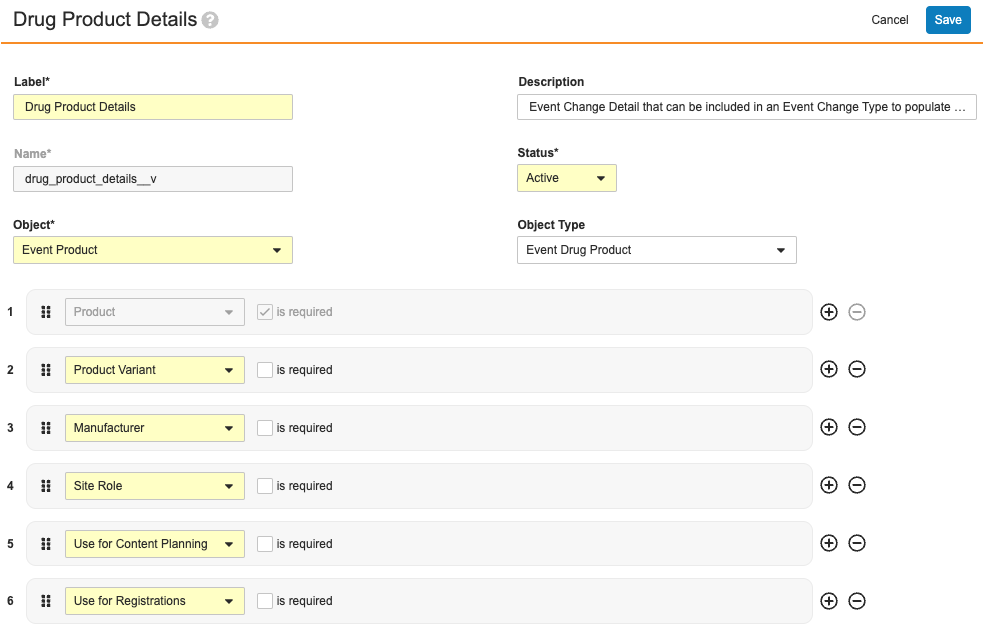
As illustrated in About Event Change Types, a single Drug Product Change Event Change Type record includes:
- One required Event Change Details record, Drug Product Details
- 11 optional Event Change Details records.
Of these, each defines their own set of required and optional fields. For example, the Drug Product Details record shown below specifies that the Product field is required, and the remaining are optional.
Note: When a given field is required for an Event relationship object, the field is automatically required when configuring a corresponding Event Change Detail. Conversely, when an Event Change Detail record requires a field that the object field configuration defines as optional, the wizard requires the field according to the Event Change Detail.
Managing Event Change Types & Details
While your organization may require additional Event Change Types or Event Change Details, Veeva provides sample records as a starting point. The sections below discuss general guidelines for these records, all of which apply when updating the delivered records or creating your own.
Note: When creating new Event Change Types, you must first create at least one corresponding Event Change Detail, as this is a required field.
Navigation
Event Change Types and Event Change Details are both located in Admin > Configuration > Application Configurations > Registrations Setup:
- The Event Change Types - View and Event Change Details - View options display all such existing records. Select a record’s Name, then Edit from the record’s Actions menu, or click + Create to create a new record.
- The Event Change Types - Create and Event Change Details - Create options allow you to immediately create that record.
Working with Event Change Types & Details
Except where indicated, both record types require or allow you to populate the following fields:
- The Label and Name are required for identifying the record in the Admin UI. You cannot update the Name field after saving the record. Optionally, add a Description for additional optional clarity on the record’s use in your Vault’s configuration.
- The Status field must be set to Active in order for the wizard to consider the record.
- For Event Change Types only:
- The Related Change Type fields (Add, Withdraw, Replacing, and Replaced By) correspond to the Related Change Type picklist. You can re-label this picklist’s standard values, however you cannot deactivate or create custom ones.
- The Primary Event Change Detail references a single Event Change Detail record.
- Once you’ve populated the record’s required fields, Vault pre-populates the Primary Event Change Detail and its sibling Change Detail records. Select the is required checkbox to require that the referenced Change Detail is processed in the wizard.
- For Event Change Details only:
- The Object dropdown includes all Event relationship objects, and when applicable, the Object Type dropdown includes the selected object’s object types.
- Once you’ve populated the record’s required fields, Vault pre-populates the selected object or object type’s required fields, according to their field configuration. When field configuration does not take precedence, you can select or deselect the is required checkbox. When a field is required in the Event Change Type record, users must complete this field when using the wizard.
Related Permissions
Admin Permissions
In order to set up Event Change Type and Event Change Detail records, Admins must have a permission set with Metadata API permission, located in the permission set’s Application tab.
Admins without this permission receive a Page Not Found error when attempting to work with Event Change Types or Event Change Details in Admin > Configuration > Application Configurations > Registrations Setup.
User Permissions
Event Object Actions
Users must be assigned to a role with Execute access in all applicable Event lifecycle states for the following actions:
- Create Event Details
- Manage Event Details
- Review Event Details
- Clear Preview
Objects, Object Types, and Fields
Vault presents data to users according to their permissions for the objects, object types, and fields referenced in the underlying Event Change Type and Event Change Detail configuration records.
For example, the Active Substance Change Event Change Type and its primary Event Change Detail (Active Substance Details) specify the Event Active Substance object’s Active Substance field as required. This means a user’s permission set must include, at minimum:
- Read permission for the Event object and its applicable object types
- Create permission for the Event Active Substance relationship object
- Edit permissions for the Active Substance field in the Event Active Substance object.
About Relationship Objects
All Vault-generated and -managed event details are considered to be Event relationship (or “join”) object records. Each Event relationship record includes the relevant data for a particular event. For example, an Event Shelf Life or Condition record includes the Product, Product Variant, Packaging, Shelf Life, Storage Condition, and other information for the Event record to which it is related.
This logic similarly applies to the Submission and Regulatory Objective relationship records referenced by this and the Create Related Records wizard.
Note: Event relationship records are distinct from the Event Change Type and Event Change Detail configuration records required to create them. See About Event Change Types & Details for more information.
Relationship Object Inventory
The list below details all objects which are related or joined to the Event object. References to an Event relationship or detail record on this page refer to any such record in the list, unless otherwise specified.
For example, the Active Substance object is related via the Event Active Substance object found in Admin > Configuration > Objects.
- Active Substance
- Authorization
- Change Item
- Clinical Study
- Inactive Ingredient
- Indication
- Nonclinical Study
- Packaging
- Packaging Characteristic
- Pharmaceutical Form
- Product
- Product Characteristic
- Nomenclature Code (
classification__v) - Regulatory Text
- Shelf Life or Condition (
shelf_life__rim) - Site Contact
- Site Organization
- Site Role
This feature also uses the Event Change Item Labeling Concept relationship object, relating Event Change Items to Labeling Concepts.
Similarly, this inventory applies to Submission and Regulatory Objective relationships, for example Submission Active Substance and Regulatory Objective Active Substance.