Vault can aggregate Identification of Medicinal Products (IDMP) data for you, offering a simple way to compile product data before submitting it to the European Medicines Agency (EMA). Vault uses a series of algorithms to compile existing submission data in your RIM Registrations Vault into the IDMP object structure.
RIM Registrations Vaults include all IDMP objects and the IDMP aggregation algorithm automatically. You must perform the configurations in this article for users to work with IDMP data. See Generating IDMP Data for detailed information about generating the IDMP elements.
Note: This feature is only available on RIM Registrations Vaults. IDMP is an evolving functionality and we will continue to add features in future releases.
Configuration Overview
To set up IDMP data review:
- Configure the Generate IDMP Records action on the Regulatory Objective object lifecycle. See details about configuring this action below.
- Create an IDMP Product Data Submission object lifecycle and associate it with the IDMP Product Data Submission object.
- Configure the Generate IDMP Elements user action on the IDMP Product Data Submission object lifecycle. You can’t assign this action to the Active state; instead you must create a new lifecycle state on which to add this action.
- Configure the necessary object and object type page layouts.
- Set necessary shared document fields to Active and associate them with any document types that you might use as attachments on the IDMP report.
- Load the necessary controlled vocabulary files to support IDMP data.
- Optional: Enable IDMP and UDI accelerators. These allow Vault to automatically generate related records for certain IDMP objects. We also recommend configuring the Link Packaging to MP-Registration action on the Registered Packaging object lifecycle.
- Optional: Configure the Pending Withdrawal lifecycle state type to allow Vault to exclude source records from IDMP data aggregation.
- Optional: Activate object types and configure object page layouts to support regulatory text with multiple translations.
- Enable and configure eAF output reports to allow users to generate variation and product reports for working with eAF web forms in the EMA portal, then view them in the IDMP Viewer.
- Optional: Configure FHIR message generation and export
- Optional: Configure PMS submission capabilities.
- Optional: Enable various IDMP Viewer enhancements.
Configuring IDMP Record Generation
The Generate IDMP Records action allows Vault to generate all IDMP data related to a Regulatory Objective record’s registrations at once. You can configure this as either a user action or an entry action on Regulatory Objective object lifecycle states. Do not configure this as an event action, or Vault will not be able to create records.
If you are configuring this as a user action, you must first assign the Generate IDMP Records object action to the Regulatory Objective and Product Data Submission objects. Do not select the Available in All Lifecycle States checkbox when you assign the object action. Instead, we recommend configuring user actions such that:
- On the Regulatory Objective lifecycle’s Planned state, users can only execute it when the Application Region equals “European Union” and the Applicable Product Type equals “Drug”.
- On the Regulatory Objective lifecycle’s In Progress State, the Applicable Product Type equals “Drug”.
- On the Product Data Submission lifecycle’s Planned state, users can always execute the action.
In order for this action to run successfully, you must also:
- Assign the IDMP Job ID field to the Regulatory Objective object’s Regulatory Objective object type.
- Ensure that there are no required fields configured on the IDMP Product Data Submission object.
Object Page Layouts
The RIM data model is periodically updated to better support evolving IDMP needs and new feature functionality.
For the 24R3 release and earlier, many of these changes were incorporated into a configuration workbook as a resource for aligning RIM Vaults to the IDMP data model. This workbook and its instructions are preserved below, covering updates for applicable releases from the 20R3 general release through the 24R3 general release.
As of 25R1, this workbook is no longer maintained. Instead, RIM Vault Admins should consult that release’s Data Model Documentation workbook in Veeva Connect to assess any IDMP-specific RIM data model updates.
24R3 General Release & Earlier
Note: This workbook is no longer maintained as of the 25R1 general release.
The IDMP data model object page layout configuration workbook details the object page layout configurations available to align your Vault to the IDMP data model in 24R3 and earlier.
The Related Object Sections tab details the source object page layouts requiring related output object sections. The Object Fields tab details which fields you can add to or remove from the indicated object’s page layout.
In both tabs, you can also:
- Consult the Additional Instructions column for configuration considerations, which can include information about the fields Vault automatically adds to object page layouts.
- Filter the Release column to determine when a given component was added or last updated.
The Object Fields tab also details whether an object contains source or output data, as this may determine your organization’s configuration requirements. If your organization’s data management strategy requires submission data to be edited at the source, you may consider omitting term and term text fields from the corresponding output records, such as Legal Basis Term and Legal Basis Term Text from the IDMP Classification System object.
About the Use for XEVMPD/IDMP Field
In some situations, you may want Vault to ignore certain Country Language records related to the submission when generating IDMP data. When this field is configured on the Country Language object page layout, users can indicate that Vault should ignore specific records by setting the Use for XEVMPD/IDMP field to No on each Country Language record. When the field is blank or set to Yes, Vault includes the record when generating IDMP data.
Supporting Regulatory Text with Multiple Translations
Note: The Product Variant Description object types Device Intended Use and Device Description related to this function are no longer needed as of 23R3.
The IDMP data aggregation algorithm supports regulatory text records with multiple translations. To configure this in your Vault, we recommend the below configuration.
- Review the object page layout configuration workbook and configure the Related Object Section and Object Field configurations for supporting regulatory text with multiple translations. You can find them by filtering the Release column to 23R3 and reviewing the Additional Instructions column.
- Ensure the Device Description, Device Trade Name, and Device Intended Use object types are active within the below objects, then create a corresponding page layout for each:
- Regulatory Text
- Regulatory Text Translation
- Event Regulatory Text
- Application Regulatory Text
- Submission Regulatory Text
- Regulatory Objective Regulatory Text
- Registered Regulatory Text
- Optional: Configure a Regulatory Text tab to allow users to view Regulatory Text object records.
Shared Document Fields
Set the following shared document fields to Active and associate them with any document types that you might use as attachments on the IDMP element:
- IDMP Document Type
- IDMP Effective Date
- IDMP File Type
- IDMP PMS ID
- IDMP PMS URL
- Medicinal Product
Controlled Vocabularies
Vault uses Controlled Vocabulary records to populate certain fields on IDMP Product Data Submission records. These records must be in place before users can begin the IDMP data collection and review process.
RIM Registrations Vaults include Controlled Vocabulary records as part of the Veeva Services implementation process. Once established, Controlled Vocabulary records for use in IDMP should be maintained using the EMA RMS Integration. Generally, these records must have the appropriate SPOR Term and *SPOR Referential ID *(spor_rms_id__v) field values to ensure that Vault can determine critical aggregation and validation criteria.
Vault supports Controlled Vocabularies for all types available within the Controlled Vocabulary Type picklist. Veeva periodically delivers new standard types via documented data model changes for a given release.
Note: When a Controlled Vocabulary record does not include SPOR Term and SPOR Referential ID (spor_rms_id__v) field values, users generating IDMP records receive configuration errors in the resulting CSV file. These errors may be referencing either the record’s missing SPOR data, or the absence of the SPOR Term at the source.
Centralised Procedure Controlled Vocabulary Requirements
To ensure that Vault can generate IDMP elements correctly and automatically create Medicinal Product and Medicinal Product Registration records, you also need to update the Centralised Procedure controlled vocabulary:
- Navigate to Admin > Business Admin > Controlled Vocabularies.
- Search to find the Centralised Procedure Controlled Vocabulary record in the grid.
- Select Edit Columns from the grid’s Actions menu and add the SPOR RMS ID and Veeva RIM UUID fields as columns.
- Using inline editing, set the SPOR RMS ID field to 100000155059. Set the Veeva RIM UUID field to 7aa0df44-87b9-4309-91a5-20185cdd2415.
Enabling IDMP Accelerators
To enable IDMP accelerators, navigate to Admin > Settings > Application Settings and set the Enable IDMP and UDI Accelerators checkbox. Then, select the checkboxes for the accelerators that you want to enable:
- Automatically Generate Medicinal Product Records: When users create new Marketed Drug Product Registration records, Vault automatically creates related Medicinal Product and Medicinal Product Registration records. After setting this checkbox, select:
- The Regions for which Vault should create records, for example, European Union.
- The appropriate Lead Market Field, or the Application object field that indicates the rapporteur for centralised procedures.
- Any Eligible Registration Types for which Vault should create records. This subsetting is optional and can be used according to your organization’s requirements, such as for animal health products. This functionality is not currently intended for investigational products and will be supported in a future release.
- Automatically Generate Registered Packaged Product Records: When users create certain related records, Vault automatically creates Registered Packaged Medicinal Product records.
- Automatically Generate Registered Site Role Records: When users create certain related records, Vault creates Registered Site Role records automatically.
- Automatically Generate Alias Records: When users create Active Substance or Inactive Ingredient records, Vault automatically creates a corresponding Active Substance Alias (
active_substance_translation__v) or Inactive Ingredient Alias (inactive_ingredient_translation__v). You can use the RIM Maintenance tab to extract existing Active Substance and Active Ingredient records, then load new alias records. The tab also supports extracting and loading Product Manufactured Dosage Form records based on existing active Product records’ Manufactured Dosage Form field.
System-managed naming must be configured on the following objects in order for you to enable their corresponding accelerator:
- Active Substance Alias (
active_substance_translation__v) - Inactive Ingredient Alias (
inactive_ingredient_translation__v) - Medicinal Product
- Medicinal Product Registration
- Registered Packaged Medicinal Product
- Registered Site Role
See Generating IDMP Data for details about record creation. If you have any mandatory fields on these objects, they must have a default value defined or record creation will fail.
Enabling Medicinal Product Registration Mapping
After you enable the Automatically Generate Medicinal Product Records IDMP accelerator, we recommend configuring the Link Packaging to MP-Registration action as a user action or an entry action on Registered Packaging object lifecycle states. This action maps Registered Packaging records to related Medicinal Product Registration records.
If you are configuring this as an entry action, do not configure the action on the Starting state, or bulk record creation issues could occur. If you are configuring this as a user action, you must first assign the Link Packaging to MP-Registration object action to the Registered Packaging object. Do not select the Available in All Lifecycle States checkbox when you assign the object action.
Configuring the Pending Withdrawal State Type
Using the Pending Withdrawal state type, you can configure various registered details object lifecycles to exclude records from the IDMP data aggregation algorithm. This can be useful, for example, when a Registered Active Substance record is in the process of being withdrawn but whose withdrawal is not yet complete, or when a registered detail’s Full Indication Text was submitted for update but is not yet approved.
To configure this:
- Review your Vault’s available object lifecycles, then create a lifecycle for any of the desired registered details objects which do not already have one.
- Within the source object lifecycle, confirm or create the lifecycle state your organization uses to indicate a record is being withdrawn. We recommend using a Pending Withdrawal label to match the state type.
- Map the new or existing lifecycle state to the Pending Withdrawal state type.
- Where required, configure the source object lifecycle to allow Vault or Vault users to move the source object record to the state mapped to the Pending Withdrawal state type. For example, you can add a manual user action to move Registered Active Substance records in the Planned state to the Pending Withdrawal state. Ensure your chosen method aligns with any existing object lifecycle logic.
Configuring FHIR Message Generation & Export
Vault can generate discrete messages in accordance with the Fast Healthcare Interoperability Resources (FHIR) standard by transposing product data in a PDS Medicinal Product Element and its related records into a XML structure. Once generated, Vault stores the output in the Vault Library under the IDMP > EU IDMP Submission subtype. When configured, Vault additionally supports sending generated in Vault directly to PMS.
Vault supports generating and exporting FHIR messages individually from a Product Data Submission record, or in bulk from a Regulatory Objective.
Controlled Vocabularies for FHIR Version Support
Vault generates FHIR messages using FHIR v5.0.0 only. Similarly, the IDMP Viewer only displays complete comparison data for FHIR v5.0.0. See additional details about FHIR version support.
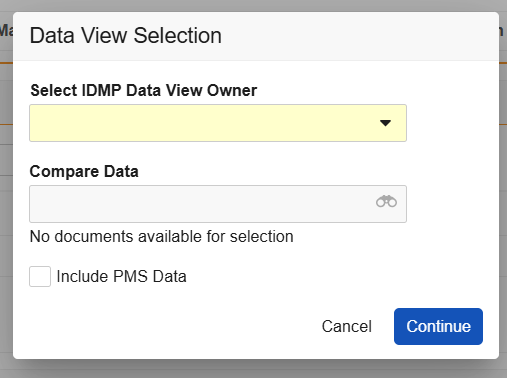
FHIR versions are reflected in your Vault’s Controlled Vocabulary records of the FHIR Message Version type. Vault populates this version within the EU IDMP Submission document’s FHIR Message Version field, which allows IDMP Viewer users to select the message from the Data View Selection dialog’s Compare Data dropdown.
Individual Generation & Export
To configure individual FHIR message generation and export:
- Ensure the IDMP > EU IDMP Submission document type is active.
- Within the Product Data Submission object, assign the Generate FHIR Message object action. During configuration, do not select the Available in All Lifecycle States configuration option.
- Within the Product Data Submission lifecycle, create a “Generate FHIR Message” user action referencing the new action. We recommend configuring the action such that users can always execute it from the lifecycle’s Planned state.
- Review your Vault’s security configuration and ensure users generating FHIR messages are assigned the appropriate permissions. Generally, users performing the Generate FHIR Message action can only generate XML for the PDS output objects to which they have Read permission.
Bulk Generation & Export
To configure bulk FHIR message generation and export:
- Within the Regulatory Objective object, assign the Bulk Create FHIR Messages and Bulk Export FHIR Messages object actions. During configuration, do not select the Available in All Lifecycle States configuration option.
- Within the Regulatory Objective lifecycle, create two new “Bulk Create FHIR Messages” and “Bulk Export FHIR Messages” user actions referencing the new actions. We recommend configuring the actions such that users can execute them from the In Progress state when the Application Region equals “European Union” and the Applicable Product Type equals “Drug”.
- Review your Vault’s security configuration and ensure users generating FHIR messages are assigned the appropriate permissions. Generally, users performing the bulk actions can only generate XML for the PDS output objects to which they have Read permission.
Configuring FHIR Message Submission to PMS
With additional Vault configuration, PMS connection setup, and baselining of IDMP records, certain submissions generated in Vault can be submitted individually or in bulk directly from Vault to PMS.
Note: This feature currently supports manufacturing and pack size enrichment submissions only.
General Configuration
- Add the Submission Status field to the Product Data Submission object page layout (
idmp_product_data_submission__v). We additionally recommend configuring security for this field such that only a Vault Admin or other higher-level users can edit it. Vault uses this picklist’s values to control various aspects of this feature (including the actions available to users), and therefore most users should not be able to edit it, however some editability should be maintained for troubleshooting purposes. - Assign this feature’s object actions to the indicated objects:
- Product Data Submission object: Submit FHIR Message and Update Submission Status actions
- Regulatory Objective object: Bulk Submit FHIR Messages action
- Within the Product Data Submission’s assigned lifecycle, navigate to the existing Create Record Event Action. Then, create a new event action such that Vault always updates the Submission Status field to “Not Submitted” when a record is created.
- Add the Connection (
connection__v) field to the Organization (organization__rim) object page layout. - Recommended: Inactivate the IDMP Submission Mode picklist’s eCTD (
ectd__v) value. This step is recommended to insure against inaccurate data entry. - Enable the appropriate IDMP Viewer Enhancement setting for viewing PMS data in the IDMP Viewer.
Note: The Product Data Submission object’s FHIR Submission URL field supports FHIR message submission response handling only. This field does not require configuration on the object page layout.
PMS Connection Setup
When a Marketing Authorization Holder (MAH) registers for SPOR API access, the EMA returns application registration details. You’ll use these details to establish your Vault’s connection with PMS.
- Navigate to Admin > Connections > Connection Authorizations and create a new Client Credential record. Populate the Name according to your organization’s requirements, and the Client ID and Client Secret field details according to the EMA-provided details.
- Navigate to Admin > Connections and create a new External connection record. Then, populate fields accordingly.
- The connection’s Name (for example, “PMS Connection”), API Name (for example,
spor_pms_api), and optional Description can be populated according to your organization’s requirements. - Set the URL to
https://spor.azure-api.net/pms/api/v3 - Select the connection Authorization record you created in Step 1.
- Do not select an Authorized Connection User.
- The connection’s Name (for example, “PMS Connection”), API Name (for example,
Configuration for Baselining IDMP Records
To support the enrichment process, IDMP records must first be baselined, or populated with all critical PMS identifiers such as the PMS ID, pack-level PMS ID, PCID, and MPID to RIM via the SPOR API. This process maps and updates the returned identifiers to the Medicinal Product and related Registered Packaged Product records, based on the Medicinal Product record’s EV Code.
To configure this capability:
- Within the Medicinal Product object, create two new object actions using the Populate PMS Identifiers and Bulk Populate PMS Identifiers actions. During configuration, do not select the Available in All Lifecycle States configuration option.
- Within the Medicinal Product object lifecycle, configure the Populate PMS Identifiers and Bulk Populate PMS Identifiers actions as user or entry actions, according to your organization’s requirements.
- When configured as an entry action, Vault can continuously sync identifiers with new records as they are created.
- Both actions should be configured such that it is only executed when the Medicinal Product record’s Authorisation Type equals Authorised Medicinal Product. As part of this configuration, ensure the referenced Authorised Medicinal Product (Controlled Vocabulary record) has a Veeva RIM UUID value of 5571e70a-8b16-42cb-924a-1f2d9d3eb41b. This is because PMS is currently limited to marketed medicinal products.
- Ensure your Vault contains the following operation- or procedure-type Controlled Vocabulary records (where Controlled Vocabulary Type is “Operation Type” or “Procedure Type”) with the appropriate Veeva RIM UUID values:
- One “Manufacturer and Pack Size Enrichment” operation type record with a Veeva RIM UUID value of 5990d2f0-338f-4169-bf80-0bab69a7ad56
- One “Full Data Set” operation type record with a Veeva RIM UUID value of a2fb662e-01b5-4af7-8e3b-5240ac6a7946
- One “Centralised Procedure” procedure type record with a Veeva RIM UUID value of 7aa0df44-87b9-4309-91a5-20185cdd2415
Enabling IDMP Viewer Enhancements
You can enable various IDMP Viewer enhancements via Admin > Settings > Application Settings. Each enhancement corresponds to an option for user selection within the IDMP Viewer’s Data View Selection dialog that appears upon running the Generate IDMP Data View action.
To enable any enhancement, you must first select the Enable IDMP Viewer Enhancements option, then select the desired subsetting(s). In most cases, subsettings can be enabled independently or together.
- Prompt for IDMP Viewer User: When enabled, users executing the Generate IDMP Data View action can select a single user to send a notification to a specific Data View Owner, with a user-specific link to an IDMP Viewer session. When disabled, the notification is sent to the user executing the action.
- Prompt for FHIR Message Selection in IDMP Viewer: When enabled, the viewer prompts users to select whether they’d like to include FHIR messages for comparison in the viewer via the dialog’s Compare Data dropdown.
- Two PMS-specific settings determine the dialog’s Include PMS Data default checkbox selection: The Default IDMP Data View to Include PMS Data setting automatically selects this checkbox, meaning Vault includes PMS data in the IDMP data view by default, unless the user deselects the checkbox (opts out). Otherwise, Enable PMS Data in the IDMP Viewer requires viewer users to select the checkbox (opt in) each time they generate a view.
Related Permissions
Users generating IDMP data must be assigned a permission set with Read, Create, and Edit permissions for the following objects:
- IDMP Grouping
- Medicinal Product
- Product Data Submission (
idmp_product_data_submission__v) - All IDMP (PDS) element objects
Generally, a user’s ability to work with IDMP data is based on object and object field permissions. This includes permission to view both lookup fields and the field it references. For example, when a user has Read permission for the PDS Medicinal Product Element object’s Domain Code and Domain Term fields, but does not have permission for the Domain field, Vault does not populate the Domain field.
PDS Element Objects
As of 25R3, Registrations Vaults include the following PDS element objects:
- PDS Admin Ingredient Manufacturer (
idmp_admin_ingredient_manufacturer__v) - PDS Admin Product Ingredient Element (
idmp_admin_product_ingredient_element__v) - PDS AP Reference Strength (
idmp_ap_reference_strength__v) - PDS Attachment (
idmp_attachment__v) - PDS Attachment Language (
idmp_attachment_language__v) - PDS Authorised Pharmaceutical Form (
idmp_authorised_pharmaceutical_form__v) - PDS Classification System (
idmp_classification_system__v) - PDS Component (
idmp_component__v) - PDS Component Material (
idmp_component_material__v) - PDS Container (
idmp_container__v) - PDS Container Data Carrier (
idmp_container_data_carrier__v) - PDS Container Material (
idmp_container_material__v) - PDS Container Reference Item (
idmp_container_reference_item__v) - PDS Device Description (
idmp_device_description__v) - PDS Device Manufacturer (
idmp_device_manufacturer__v) - PDS Ingredient Master File (
idmp_ingredient_master_file__v) - PDS Manuf. Item Ingredient Manufacturer (
idmp_manuf_item_ingredient_manufacturer__v) - PDS Manuf. Item Ingredient Master File (
idmp_manuf_item_ingredient_master_file__v) - PDS Manufactured Item (
idmp_manufactured_item__v) - PDS Manufactured Item Description (
idmp_manufactured_item_description__v) - PDS Manufactured Item Ingredient (
idmp_manufactured_item_ingredient__v) - PDS Manufacturing Site (
idmp_manufacturing_site__v) - PDS Marketing Authorisation Element (
idmp_marketing_authorisation_element__v) - PDS Master File (
idmp_master_file__v) - PDS Medicinal Product Element (
idmp_medicinal_product_element__v) - PDS Medicinal Product Full Name (
idmp_medicinal_product_full_name__v) - PDS Medicinal Product Indication (
idmp_medicinal_product_indication__v) - PDS Name Part (
idmp_name_part__v) - PDS Pack Size (
idmp_pack_size__v) - PDS Package Authorisation Status (
idmp_package_authorisation_status__v) - PDS Package Description (
idmp_package_description__v) - PDS Package Manufacturer (
idmp_package_manufacturer__v) - PDS Package Marketing Status (
idmp_package_marketing_status__v) - PDS Packaged Medicinal Product Element (
idmp_packaged_medicinal_product_element__v) - PDS Pharmaceutical Product Description (
idmp_pharmaceutical_product_description__v) - PDS Pharmaceutical Product Element (
idmp_pharmaceutical_product_element__v) - PDS Product Cross Reference (
idmp_product_cross_reference__v) - PDS PV Reference Strength (
idmp_pv_reference_strength__v) - PDS Regulatory Authorisation (
idmp_regulatory_authorisation__v) - PDS Route of Administration (
idmp_route_of_administration__v) - PDS Shelf Life (
idmp_shelf_life__v) - PDS Storage Precaution (
idmp_storage_precautions__v) - PDS Therapeutic Indication Element (
idmp_therapeutic_indication_element__v)