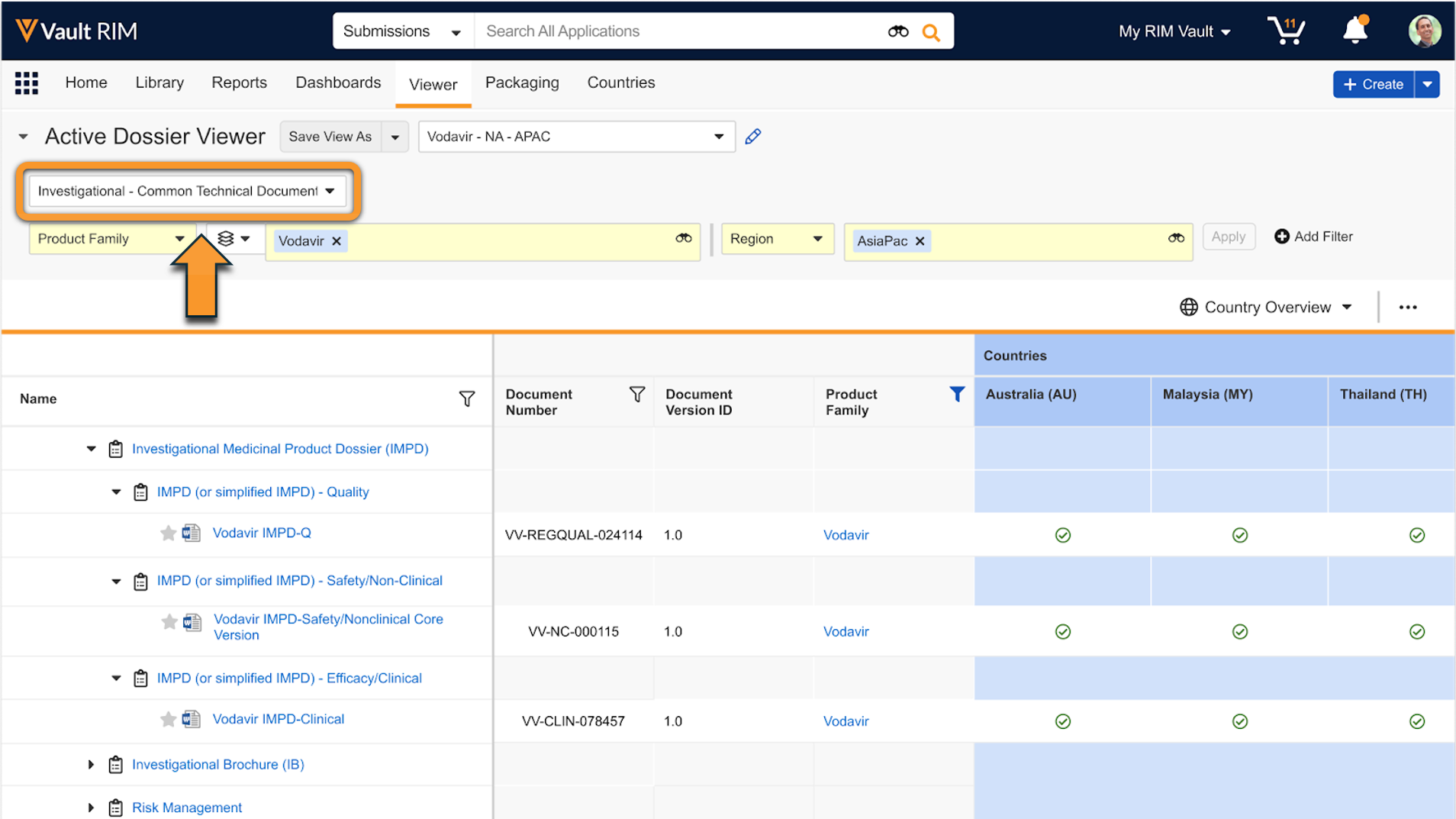
RIM Submissions and RIM Submissions Archive Vaults include the Active Dossier structure to help sponsors maintain a list of current documents for a given product and market. Users can view the Active Dossier in a designated tab to see a document’s status across multiple markets.
When configured, Vault can automatically populate the Active Dossier based on changes to Submission and Regulatory Objective records:
- When a Submission record moves to final states such as Health Authority Received or Archived state, Vault can automatically create and update Active Dossier Item and Active Dossier Item Detail records. Active Dossier Structure records may be created for newly-added Active Dossier Items.
- When a Regulatory Objective record moves to the Health Authority Approved, Rejected, Withdrawn, or On Hold states, Vault can automatically update Active Dossier Item and Active Dossier Item Detail records for all related submissions.
You can also configure Vault to generate Active Dossier records from a Global Content Plan dispatch.
Note: This feature is only available in Vaults licensed for both RIM Submissions and RIM Submissions Archive. Active Dossier generation from Global Content Plan Dispatch requires a license for RIM Registrations.
Active Dossier Objects & Components
The following objects support the Active Dossier and capture the structure, product-related metadata, and the status of a specific document within a given market or country:
- Active Dossier Template contains the template outline of the Active Dossier. Active Dossier Template records are provisioned and maintained by Veeva. Users can only edit the Status, Document Type, and Reference Model fields on these records. Vault supports a limited set of Active Dossier Template records within the Common Technical Document (CTD).
- Active Dossier Structure contains the outline of the Active Dossier built based on metadata and the Active Dossier Template. Vault creates these records automatically based on the tokens specified in the Active Dossier Template records and unique combinations related object reference fields. For example, Vault creates a “3.2.S Drug Substance - Wondersubstance” Active Dossier Structure record for Active Dossier Item records with “Wondersubstance” as the Active Substance.
- Active Dossier Item captures each individual source document and version and the current state of the document per country, as well as the product information metadata. Users cannot edit the Country or any fields related to the document.
- Active Dossier Item Detail is a child object of Active Dossier Item that captures the in-progress state and additional context of the document in a specific country. This includes the regulatory transaction related references such as the Application, Regulatory Objective, Submission, Event, and Activity. It also captures additional documents related to the instance of the document for the country, such as the Submissions Archive Document and Translation Document. Users cannot edit the Country, product information fields, or any fields related to the document.
- Active Dossier Loader is a raw object available for creating Active Dossier records for migration purposes. To expedite the initial migration in your Vault, you can use Vault Loader to load records of this object to quickly create Active Dossier Item, Active Dossier Item Detail, and Active Dossier Structure records in bulk. See Loading Active Dossier Records.
Active Dossier Objects for Registration & Product Information Tracking
The Active Dossier Item Detail object is related to the below objects, which Registrations customers can use to provide users a more granular view of the Active Dossier based on the Registration and select product information details. When configured, Vault automatically creates Active Dossier Item Detail relationship records for Registrations, Product Variants, Manufacturers, and Inactive Ingredients based on matching fields between related Registrations, Active Dossier Item Details, and document metadata.
- AD Item Detail Inactive Ingredient
- AD Item Detail Manufacturer
- AD Item Detail Product Variant
- AD Item Detail Registration
These relationships are surfaced in the Active Dossier Viewer and are editable in the Active Dossier Editor. When you edit these relationships, Vault prompts you to enter a Reason for Change. This ensures manual edits are captured in the audit trail, and aligns this workflow with other standard editing flows within Active Dossier.
Configuration Overview
- Activate the Active Dossier tab and reorder it to be a sub-tab of the Product tab.
- Activate and configure the Edit Active Dossier and View Active Dossier actions. See details about configuring these actions below.
- Create an object workflow that allows Vault to generate Active Dossier records when submission creation completes. See details about object workflow configuration for submissions below.
- Review your Vault’s Submission object lifecycle configuration. Add a Start Workflow entry action for the Generate AD records from Submissions workflow on the Health Authority Received state, and for any lifecycle states where records should be generated, for example, the Archived state.
- Review your Vault’s Regulatory Objective object lifecycle configuration. Add an Update Active Dossier entry action on the Health Authority Approved state, and any lifecycle states where the status and optionally the Approval Type of related Active Dossier records should be updated, for example Rejected, Withdrawn, or On Hold.
- Update the Regulatory Objective object page layout to add a related object section for the Active Dossier Item Details object.
- Update Active Dossier Template records to populate the Document Type field or the Reference Model field. Optionally, you can edit and add templates according to your organization’s needs. See details about updating the Active Dossier Template below.
- Review your Vault’s security configuration to ensure users can view and create Active Dossier records, as well as execute actions.
- Review your Vault’s Country Language records, and create any records for countries and languages where translations are expected. Active Dossier relies on these records for tracking translation documents.
- Recommended: Activate the Incorrect Document Type value in the Active Dossier Update Reason picklist. Depending on your organization’s requirements, you may also consider creating additional picklist values.
- Optional: Load active dossier records using Vault Loader. This step is recommended if your organization plans to migrate dossier information from an existing custom solution or an external system for tracking current documents in Vault. See Loading Active Dossier Records.
- Optional: Configure your Vault to generate Active Dossier records from a Global Content Plan dispatch.
- Optional (Registrations only): Configure Active Dossier Registration & Product Information Tracking.
Configuring Edit & View Active Dossier Actions
The Edit Active Dossier and View Active Dossier actions are available on the Submission, Regulatory Objective, and Event objects. When a user selects either action from these records, Vault filters the Active Dossier based on the record’s join relationships.
Complete these steps on the Submission, Regulatory Objective, and Event objects:
- Navigate to Admin > Configuration > Objects > {Object} > Actions.
- Edit the Edit Active Dossier and View Active Dossier actions and set the Status to Active for both.
- Navigate to Admin > Configuration > Objects > {Object} > Object Types and click Actions.
- From the Actions menu, select Edit Object Type Actions.
- Add the Edit Active Dossier and View Active Dossier actions to all object types.
- Click Save.
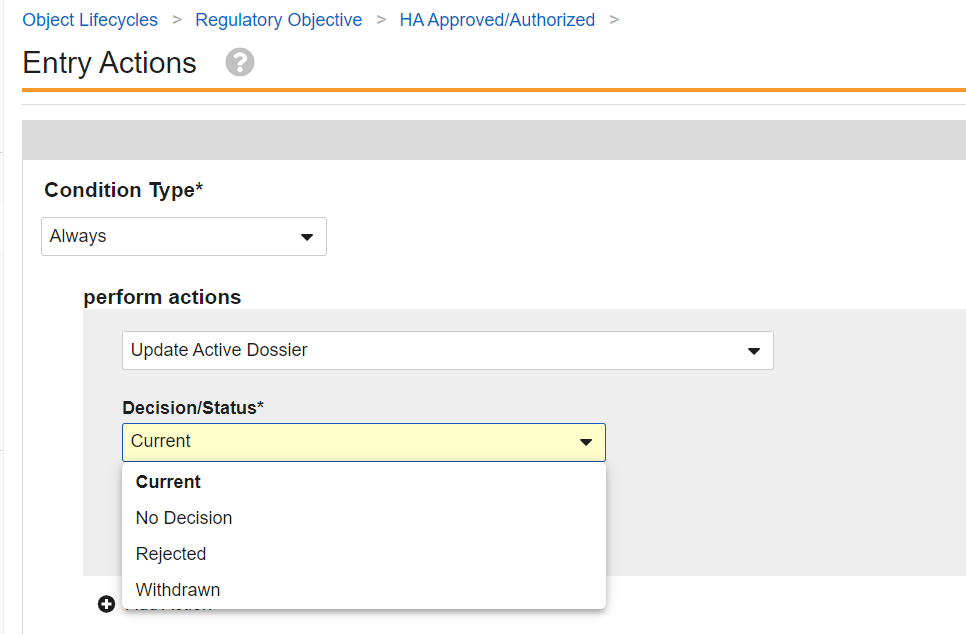
Configuring the Update Active Dossier Entry Action
The Update Active Dossier entry action is configurable within a Regulatory Objective lifecycle state. Two configuration options are available when the Update Active Dossier system action is configured.
Decision/Status
The Decision/Status picklist has the following options:
- Current
- Rejected
- No Decision
- Withdrawn
When Current is configured on the entry action:
- For each applicable Active Dossier Item Detail whose document is included in the submission’s archive or relevant content plan, Vault sets the Active Dossier Status to Pending Current.
- Only Active Dossier Item Details that meet the following conditions are updated to Pending Current:
- Active Dossier Status is Submitted.
- Metadata matches at least one of the related Regulatory Objective’s relationships. This means that it is expected for the Regulatory Objective relationships (Products, Active Substances, Inactive Ingredients) to include the same relationships from the Submission or Event used to generate the Active Dossier records that are in-scope for the calculation, for example those where Use For Content Planning is “Yes”.
- eCTD operation from the related Archive node is not Delete.
- Submission field is populated with a Submission that is related to the Regulatory Objective
- If Vault finds that there is already an Active Dossier Item Detail with a Current or Pending Current status that has a later version in the document version tree than the document included in the Submission’s Archive or Content Plan, the status for the Active Dossier Item Detail record is updated to Pending Superseded or Replaced, respectively, instead of changed to Pending Current. The status of the record for the later version in the document version tree is unchanged.
- The Approval Date is defaulted to the date set on the Regulatory Objective’s Actual Decision Date on the records updated to Pending Current.
- Vault attempts to determine superseded document versions to set the Active Dossier Status to Pending Superseded or Replaced for prior document version Active Dossier Item Detail records that meet the following conditions:
- Set with the relevant Application that the calculation occurs in
- Active Dossier Status of Current or Pending Current
- If the Active Dossier Item Detail record has a status of Pending Current, Vault sets the status to Replaced instead of updating it to Pending Superseded.
- Set with metadata matches at least one of the related Regulatory Objective’s relationships.
- Is seen as a previous document version either via version tree calculation or using the Originates From document relationship:
- Version tree calculation: Document with the same document ID and an earlier version within the document version tree than the version that is being set to Pending Current. For example, if a document with ID of 1234 and version of 5.0 is calculated as Pending Current, an Active Dossier Item Detail with ID of 1234 and version of 3.0, Active Dossier Status of Current, and that meets the above conditions is set to Pending Superseded.
- Originates From document relationship: Document that is a direct or indirect target of the Originates From document relationship by the document that is being set to Pending Current. For example, document F is set in the Originates From document relationship of document G, and document G is set in the Originates From document relationship of document H. An Active Dossier Item Detail record for document H will be set to Pending Current. An existing Active Dossier Item Detail record for document F with a Current status that meets the above conditions is set to Pending Superseded.
- The End Date is defaulted to the date set on the Regulatory Objective’s Actual Decision Date on the records updated to Pending Superseded.
When Rejected is configured on the entry action:
- Active Dossier Item Details for all Submissions associated with the Regulatory Objective where the entry action is initiated are moved to the Rejected Active Dossier Status.
* The End Date is defaulted to the date set on the Regulatory Objective’s Actual Decision Date on the records updated to Rejected.
When No Decision is configured on the entry action:
- Active Dossier Item Details for all Submissions associated with the Regulatory Objective are moved to the No Decision Active Dossier Status.
When Withdrawn is configured on the entry action:
- Active Dossier Item Details for all Submissions associated with the Regulatory Objective where the entry action is initiated are moved to the Withdrawn Active Dossier Status
- The End Date is defaulted to the date set on the Regulatory Objective’s Actual Decision Date on the records updated to Withdrawn.
Important: Vault can only use active Regulatory Objective relationships during the calculation, including active Submission Regulatory Objectives. This means that if the parent Submission or Regulatory Objective is in a lifecycle state where records are inactive, the calculation will not run.
Approval Type
When the Decision/Status is set to Current, the Approval Type is available for configuration. The Approval Type picklist has the following options:
- Explicit Approval
- Implicit Approval
- Partial Approval
The Approval Type configuration is only available if the Decision/Status is set to Current.
- When Explicit Approval is configured, the Approval Type field is set to Explicit Approval on the relevant records related to the Regulatory Objective.
- When Implicit Approval is configured, the Approval Type field is set to Implicit Approval on the relevant records related to the Regulatory Objective.
- When Partial Approval is configured, the Approval Type field is set to Partial Approval on the relevant records related to the Regulatory Objective.
eCTD Submissions
- For eCTD submissions with a Leaf Operation of Replace, Vault locates the target document of the Replace operation and sets the status of the Active Dossier Item Detail record for that document as Pending Superseded as long as the target document’s Active Dossier Item Detail record has a status of Current, the same Submission Metadata, and the same Application. If the target document’s Active Dossier Item Detail record has a status of Pending Current, Vault sets the status to Replaced.
- For eCTD submissions with a Leaf Operation of Delete, Vault locates the target document of the Delete operation and sets the status of the Active Dossier Item Detail record for that document as Pending Deprecation as long as the target document’s Active Dossier Item Detail record has a status of Current, the same Country, and the same Application. If the target document’s Active Dossier Item Detail record has a status of Pending Current, Vault sets the status to Replaced.
Configuring Regulatory Objective Lifecycle Update Active Dossier Entry Actions
The Update Active Dossier entry action automates the status change of Active Dossier records to Pending Current/Superseded/Deprecated, Rejected, Withdrawn, or No Decision. The entry action can also be configured to set the Approval Type when updating records to Pending Current. To configure this entry action:
- Navigate to Admin > Configuration > Object Lifecycles > Regulatory Objective.
- Configure an Update Active Dossier entry action on a Regulatory Objective lifecycle state and select the corresponding Decision/Status.
- If No Decision, Rejected, or Withdrawn is selected, then all valid Active Dossier Item Detail records related to the Regulatory Objective are moved into that configured status. If Current is selected, then the pending Current/Superseded/Deprecated calculation is run. For example, Vault moves valid Active Dossier records related to the Regulatory Objective to Pending Current, and previous superseded Active Dossier Item Details to Pending Superseded.
- If the Decision/Status is set to Current, then the Approval Type is displayed/available for configuration.
- Click Save.
Configuring Active Dossier Workflows
Configuring an Active Dossier Workflow for Submissions
Vault includes a system action that generates Active Dossier records automatically after the submission lifecycle state change is applied. Configure a new object workflow that includes this system action as follows:
- Navigate to Admin > Configuration > Workflows and click Create.
- Select the Object Workflow type in the Create Workflow dialog and click Continue.
- Enter a Label. We recommend Generate AD records from Submissions.
- In the Lifecycle field, select Submission Lifecycle.
- Set the Allow auto-start from entry action and event action checkbox.
- Click Save to save the workflow and begin adding steps.
- Create a Generate AD Records step and select the System Action type.
- In the System Action field, select Populate the Active Dossier.
- Click Save.
- Activate the workflow.
Disabling Submissions Archive Path from Active Dossier Generation
You can use the Disable Archive Population Path setting on the Populate Active Dossier workflow system action to only search the Content Plan documents and metadata to create records. If you select the Disable Archive Population Path setting, Vault disregards the documents associated in the Source References relationship on the Submissions Archive documents when populating the Active Dossier.
If you are using Veeva Submissions Publishing, the published Archive document and related Archive node are set based on the Published Document on the Content Plan Item. If a Content Plan is not published with Veeva Submissions Publishing, then the Archive fields will not be set on the records created from the Content Plan.
Enabling Active Dossier Global Tracking
You can use the Enable Active Dossier Global Tracking setting on the Populate Active Dossier workflow system action to update Active Dossier records created from Global Content Plan Dispatch from Dispatched to Submitted status when the same document version is submitted in a Submission Content Plan, without
product or study metadata comparison. When Enable Active Dossier Global Tracking is de-selected, Active Dossier records created from dispatch are updated from Dispatched to Dispatched, Not Submitted when the same document version is submitted in a Submission Content Plan and its submission relationships do not match those of the dispatched record.
Configuring Active Dossier Generation from a Global Content Plan Dispatch
When Global Content Plan creation and dispatch are configured in your Vault, enable the Populate Active Dossier action to generate Active Dossier records from a dispatch.
- In the Content Plan lifecycle, update the configured Dispatch Global Content Plan user actions to use the Populate Active Dossier configuration option. When following the recommended configuration for Content Plan Dispatch, these actions appear in the Draft and Baselined states.
- In the Event lifecycle, update the configured Dispatch Global Content Plan user actions to use the Populate Active Dossier configuration option. When following the recommended configuration for generating Global Content Plans, these actions appear in the Planned, In Progress, In Regulatory Assessment, and Regulatory Assessment Complete states.
- Optional: Use the Active Dossier Loader object to set the relevant Event, Activity, and Needs Submission values in existing Active Dossier Item Detail records.
Note: For optimal feature performance, we recommend enabling Attachments on the Submission and Event objects, as well as enabling the Enable Content Plan Comparison Application Setting. Otherwise, Active Dossier records may generate inconsistently from the Global Content Plan dispatch.
About the Populate Active Dossier Action
During configuration, Vault lists disposition scenarios with defaulted Local Disposition picklist values. Each disposition corresponds to the Local Disposition value set on the target Activity, and Vault references the value when creating Active Dossier records. Custom disposition picklist values can additionally be configured in the appropriate category. Active Dossier records will only be created for Activities with a Local Disposition that is configured in one of the disposition categories.
While dispatch and Active Dossier generation logic allow you to relabel the default Local Disposition values, we do not recommend rearranging the defaulted values for each scenario.
Configuring Active Dossier Registration & Product Information Tracking
Registration tracking provides Registrations customers with a more granular view of the Active Dossier based on the Registration and select product information details.. With the below configuration and data setup, Vault automatically creates Active Dossier Item Detail relationship records for Registrations, Product Variants, Manufacturers, and Inactive Ingredients based on matching fields between related Registrations, Active Dossier Item Details, and document metadata. These relationships are surfaced in the Active Dossier Viewer and are editable in the Active Dossier Editor.
To configure and set up data for registration and product information tracking:
- Update the Active Dossier Item Detail object page layout to include related object sections for the following objects:
- AD Item Detail Inactive Ingredient
- AD Item Detail Manufacturer
- AD Item Detail Product Variant
- AD Item Detail Registration
- Update the Active Dossier Template object page layout to include the Registered Product Information Scope field. You’ll populate this value on records later in the configuration process.
- Optional: Update the Controlled Vocabulary object page layout to include the Exclude from Active Dossier field. You’ll populate this value on records later in the configuration process.
- Optional: Update the Country object page layout to include the Exclude from Active Dossier Generation and Match on Procedure Type Countries fields. You’ll populate these values on records later in the configuration process.
- Recommended: Activate the Missing Registration Data value in the Active Dossier Update Reason picklist. Depending on your organization’s requirements, you may also consider creating additional picklist values.
- Review your Vault’s object lifecycle configuration for the objects listed below. In order to create a corresponding Active Dossier Item Detail relationship record, this feature requires that all listed objects have a lifecycle, and that the lifecycles’ state types are mapped to a lifecycle state. To support this, you can create your own state type, or add this feature’s included Pending Approval and Planned state types. Note: When configuring or reviewing state types, ensure the same types are used across all lifecycles. For example, if you updated the Registration lifecycle to use the Planned state type for this feature, the remaining lifecycles must also use the Planned state type. Otherwise, you will not be able to select the state type later in the configuration process.
- Registration
- Registered Product
- Registered Active Substance (
registered_active_ingredient__v) - Registered Inactive Ingredient
- Review your Vault’s Active Dossier Template records. For each relevant record, ensure the Registered Product Information Scope field is set appropriately for each template section. You can expedite this process using a Veeva-recommended Vault Loader file.
- Review your Vault’s Controlled Vocabulary records for manufacturing site roles (where Controlled Vocabulary Type is “Manufacturing Site Role”). For each site role Vault should ignore during Active Dossier record creation, set the Exclude from Active Dossier field to “Yes”.
- Review your Vault’s Constraint and Country records. For each Procedure Type Country Constraint mapping a country to the relevant Procedure Type, set the related Country record’s Match on Procedure Type Countries field.. This step may require creating additional Procedure Type Country Constraint records. If applicable according to your organization’s requirements, also set the Exclude from Active Dossier Generation field on the relevant Country records. See additional details below.
- Review your Vault’s security configuration for Registrations functionality. To use the Active Dossier for registration and product information tracking, users must be assigned a permission set with permissions for the Active Dossier generally, in addition to feature-specific objects and fields.
- Enable the Active Dossier Registration Tracking application setting, then select the setting’s Valid Registration State Types you configured or confirmed in Step 6. If you do not see the expected state types, confirm the same state types are used across all impacted lifecycles.
Updating Country Records
Vault uses Country records and their Exclude from Active Dossier Generation and Match on Procedure Type Countries field values to determine which Active Dossier records to create for registration tracking.
As the values you select within Country records depend on your organization’s processes, consider the below guidelines.
- If your organization’s processes use a single European Union country on an application and submission for centralised procedures, consider setting Match on Procedure Type Countries to “Yes” on the European Union Country record. This also requires creating Procedure Type Country Constraint records accordingly for the centralised procedure type.
- If your organization tracks the granular EU countries on an application and submission (instead of only a single EU country), consider setting Exclude from Active Dossier Generation to “Yes” on the European Union Country record. This prevents Vault from creating Active Dossier records for this country when populating the Active Dossier.
- For additional Country records for which it does not make sense to track within the Active Dossier (such as Common (EU) or Common (GCC)), consider setting the Exclude from Active Dossier Generation on these records to “Yes”. This prevents Vault from creating Active Dossier records for these countries when populating the Active Dossier.
About the Display Multiple Field
This feature includes the Display Multiple field, which allows Vault to indicate to Active Dossier Viewer users that a single Active Dossier Item Detail record is associated with a Registration, Product Variant, Manufacturer, and/or Inactive Ingredient when hovering over the viewer’s Multiple icon.
To do this, Vault runs the Active Dossier Display Update SDK job to set the field accordingly when any such relationship records are created or deleted. As a result, viewer users may experience a delay in viewing the Multiple icon if they are attempting to do so before the update job completes its run.
This system-managed picklist field does not require configuration on any object page layouts.
Working with Active Dossier Templates
You can configure Active Dossier Template records and customize standard Active Dossier Templates specific to your organization’s needs. The sections below provide the recommended configuration for managing the existing records in your Vault.
Updating Active Dossier Templates
Note: For Veeva to properly maintain Active Dossier records, you cannot delete standard Active Dossier Template records, nor update a standard record’s RIM UUID Reference Model fields.
The Active Dossier Template object includes the Document Type and Reference Model fields. Vault uses these fields to determine which documents to add to the Active Dossier.
By default, Vault references the value in the Document Type field to determine the document types to include in the Active Dossier structure. You can populate the Document Type field by navigating to Business Admin > Active Dossier Template and assigning a Document Type to each record.
If the Document Type field is blank, Vault instead references the value in the Reference Model field when creating the Active Dossier Structure. The Active Dossier Template records provisioned by Veeva include values in the Reference Model field by default, but you must configure the RIM Reference Model in order for Vault to use it when creating the Active Dossier. Standard Active Dossier Templates are managed by Veeva and have a Vault RIM UUID set. The RIM Reference Model field on standard templates is non-editable.
It is recommended to set the Document Type in the RIM Reference Model. You can choose to only set the document type on the Active Dossier Template field, which does not override anything mapped in the RIM Reference Model.
See How Active Dossier Uses the RIM Reference Model for more information.
Customizing Standard Active Dossier Templates
You can customize the following on standard Active Dossier Templates to align with your organizational terminology and specific business requirements.
- The Displayed Section Name (
display_name__v) field cannot be updated for standard Active Dossier Templates that contain tokens. - Reorder Active Dossier Template records within a single level in the hierarchy.
- Incorporate new template records to track additional documents specific to your organization’s needs.
Updates made to templates are automatically synced to the Active Dossier records. There may be a delay between when the template change is made and when the change is reflected in the Active Dossier records and viewer, and therefore the sync may not be immediate.
Creating Custom Active Dossier Templates
You can create new templates within standard hierarchies, or create new template hierarchies to accommodate diverse product types or business needs.
Limitations
The following limits apply to the configuration of Active Dossier Templates:
- No more than 500 custom nodes per hierarchy.
- No more than 20 levels deep.
- Only one user can edit Active Dossier Templates within an Active Dossier Hierarchy at a time.
- Active Dossier Templates must be created level by level if they are created in bulk, for example with Vault Loader.
- Only Active Dossier Templates in the same template hierarchy can be loaded in the same batch.
- Reparenting Active Dossier Templates under a different template hierarchy is not supported.
- Updating display names of standard templates with tokens and creating custom Active Dossier Template records with tokens in the display name are not supported.
Reordering
You can reorder Active Dossier Template records by selecting a reposition action from the Actions menu for the record you want to move.
To reorder Active Dossier Template records using the Actions menu:
- Navigate to the Root or relevant Active Dossier Template. Click the View Tree Layout button to navigate to the template viewer. Ensure that Display Children and All Objects are selected.
- Open the grid item Actions menu for the record you want to move.
- Select an action under Reposition, for example Move to Bottom to reposition as the last item in the list. If you select Set Exact Position, the Set Position of Record Within List dialog opens. Enter a position for your record. The position is a number representing the place in the order of all records in the list. For example, entering 3 places the record third in the list.
- Click OK. Vault reorders the object records.
Reparenting
You can reparent custom Active Dossier Template records. You can only reparent within the same Active Dossier Template.
Configuring Multiple Hierarchies
When there are multiple Active Dossier hierarchies, the Active Dossier Viewer’s global filters include a hierarchy selector.
You must enable and configure permissions on the Root Template and Active Dossier Template fields specified and disable uniqueness on the Active Dossier Template’s Document Type field.
To use the Active Dossier Templates and multiple Active Dossier hierarchies:
- Configure the Active Dossier Template tab to allow users to create records.
- Activate the Active Dossier Template field on the Application Object and add to relevant page layouts, for example Content Plan and Publishing layouts. You must have permissions for the Active Dossier Template field and Active Dossier Template, or the viewer will fail to load.
- Activate the inactive Active Dossier templates you want to use, or create your own.
- Enrich your existing Application records to reference the relevant Active Dossier Template. If this field is blank, the existing CTD template currently in use is defaulted.
We recommend updating the Name on existing Active Dossier Template records to match the Display Name for easier readability of the Root record when the multiple hierarchy selector is displayed in the Viewer. This includes renaming the existing Root record for CTD with the Vault RIM UUID of f2d76f48-91bc-4a31-b254-3cdaaa81fc14 so that the hierarchy selector displays that Root name instead of the current auto-numbered string, for example “ADT-000089”.
Loading Active Dossier Records
If your organization is adopting Active Dossier to replace an existing custom solution or an external system for tracking submitted documents in Vault, you can use the Active Dossier Loader object to generate the necessary records. We recommend leveraging Vault Loader to create Active Dossier Loader records in bulk.
Once you’ve migrated source documents into your Vault Library, prepare a CSV input file containing the required and optional object fields to load to the Active Dossier Loader object.
When you load data to the Active Dossier Loader object, Vault uses it as an intermediate staging object to assess the standing of Active Dossier Item, Active Dossier Item Detail, and Active Dossier Structure object records in your Vault. Following the assessment, Vault creates or updates these records according to the input file.
The CSV output file Vault sends once the load is complete does not include the Active Dossier Item, Active Dossier Item Detail, and Active Dossier Structure records it created based on the Active Dossier Loader object data. You can confirm these records directly in your Vault, or extract the data from each object using Vault Loader.
The sections below outline the Active Dossier Loader required and optional object fields to include in your CSV input file, as well as the target objects and fields Vault creates, populates, and updates during the loading process.
Required Fields
The table below describes the fields required to load to the Active Dossier Loader object.
Note: To prevent invalid metadata combinations, only one of the fields marked with an asterisk (*) can be populated. For example, the Active Substance field should remain blank when the Product field is populated.
| Active Dossier Loader Field | Target Object(s) | Target Field(s) |
|---|---|---|
Source Document (document__v) |
Active Dossier Item, Active Dossier Item Detail | ADI: Document, Document Number, Document Title, Document Version ID ADID: Document, Document ID, Document Major Version, Document Minor Version |
Country (country__v) |
Active Dossier Item Detail | Relevant country field |
Active Substance* (active_substance__v) |
Active Dossier Item | Active Substance |
Product* (product__v) |
Active Dossier Item | Product Note: If the Inactive Ingredient field is populated, Product must also be populated. |
Product Family* (product_family__v) |
Active Dossier Item, Active Dossier Item Detail | ADI: Product Family ADID: Product Family Note: If an existing record is already found, specific fields are set based on the values provided. |
Root Template (root_template__v) |
Active Dossier Template | Root Template If the Template field is blank, the Root Template field is required and determines which hierarchy to create the Active Dossier records for. If the Root Template field is blank, it defaults to the existing Marketing CTD hierarchy. |
Clinical Study (clinical_study__v) |
Active Dossier Item | Note: If Indication is also set, then the Clinical Study section is created under the respective Indication section. Note: Study Type and Study Subtype are used to determine which repeating section to generate Active Dossier records under |
Nonclinical Study (clinical_study__v) |
Active Dossier Item | Nonclinical Study Type and Nonclinical Study Subtype are used to determine which repeating section to generate Active Dossier records under |
About XML Fields
The following fields are used to locate the correct Submissions Archive node and are required when there is more than one valid node for the Application and Submission, for example when a document is used in multiple sections within the same Submission:
- XML Product (
xml_product__v) - XML Manufacturer (
xml_manufacturer__v) - XML Product Dosage Form (
xml_product_dosage_form__v) - XML Drug Substance (
xml_drug_substance__v) - XML Excipient (
xml_excipient__v) - XML Clinical Study ID (
xml_clinical_study_id__v) - XML Indication (
xml_indication__v) - XML Nonclinical Study ID (
xml_nonclinical_study_id__v)
Optional Fields
The table below describes fields that are optional to include in the loader file.
| Active Dossier Loader Field | Target Object(s) | Target Field(s) | Additional Considerations |
|---|---|---|---|
Active Dossier Status (active_dossier_status__v) |
Active Dossier Item, Active Dossier Item Detail | Active Dossier Status | |
Active Substance Manufacturer (active_substance_manufacturer__v) |
Active Dossier Item | Active Substance Manufacturer | When populated, Active Substance must also be populated. |
Activity (activity__v) |
Active Dossier Item Detail | Activity | When enabling Active Dossier generation from a Global Content Plan, use this field to set a related Activity. |
ADID External ID (adid_external_id__v) |
Active Dossier Item Detail | ADID External ID | |
Application (application__v) |
Active Dossier Item Detail | Application | |
Approval Date (approval_date__v) |
Active Dossier Item Detail | Approval Date | This date is automatically populated or updated with the Regulatory Objective record’s Actual Decision Date when an ADID record is moved to a “Pending Current” status, or if an existing (Pending Current, Current) Active Dossier Item Detail is found during Regulatory Objective calculation. |
Approval Type (approval_type__v) |
Active Dossier Item Detail | Approval Type | |
Comments (comments__v) |
Active Dossier Item Detail | Comments | Free-text field that allows you to add notes or additional context about the record. |
Dispatch Date (dispatch_date__v) |
Active Dossier Item Detail | Dispatch Date | This date is automatically recorded when an Active Dossier Item Detail (ADID) is initially created from a GCP dispatch. You may inactivate this field if your organization does not use Global Content Plans. |
End Date (end_date__v) |
Active Dossier Item Detail | End Date | This date is automatically set with the superseding Regulatory Objective’s Actual Decision Date when an Active Dossier Item Detail is calculated as Pending Superseded or Pending Deprecated. It is automatically set if an Active Dossier Item Detail is moved to Rejected or Withdrawn via a Regulatory Objective state change. |
Event (event__v) |
Active Dossier Item Detail | Event | When enabling Active Dossier generation from a Global Content Plan, use this field to set a related Event. |
Inactive Ingredient (inactive_ingredient__v) |
Active Dossier Item | Inactive Ingredient | When populated, the Product field must also be populated. See also Required Fields. |
Inactive Ingredient Manufacturer (inactive_ingredient_manufacturer__v) |
Active Dossier Item | Inactive Ingredient Manufacturer | When populated, Inactive Ingredient must also be populated. |
Indication (indication__v) |
Active Dossier Item | Indication | |
Latest for Authoring (latest_for_authoring__v) |
Active Dossier Item Detail | Latest for Authoring | When this is left blank or not included, Vault will automatically set this value. This may also impact the Active Dossier records from a Latest for Authoring value on Active Dossier Item Details for earlier versions of this document version. |
Latest for Authoring End Date (latest_for_authoring_end_date__v) |
Active Dossier Item Detail | Latest for Authoring End Date | |
Needs Submission (needs_submission__v) |
Active Dossier Item Detail | Needs Submission | When enabling Active Dossier generation from a Global Content Plan, the “Yes” picklist value denotes records with dispositions requiring submission before implementation. |
Product Manufacturer (product_manufacturer__v) |
Active Dossier Item | Product Manufacturer | When populated, Product must also be populated. |
Product Variant (product_variant__v) |
Active Dossier Item | Product Variant | When populated, Product must also be populated. |
Published Report Document (published_report_document__v) |
Active Dossier Item Detail | Published Report Document | If the in-scope document as Published by RLCP set to Yes, then this document version is set within the Published Report Document field on the Active Dossier Item Detail and the source documents found in the Source References relationship are used for Active Dossier record creation. |
Regulatory Objective (regulatory_objective__v) |
Active Dossier Item Detail | Regulatory Objective | |
Submission (submission__v) |
Active Dossier Item Detail | Submission | |
Submissions Archive Document (archive_document__v) |
Active Dossier Item Detail | Submission Metadata, Submissions Archive Document, Is eCTD | When populated, Application and Submission must also be populated. In some cases, XML fields must also be populated in order to locate the correct Submission Archive node. See About XML Fields. |
Submitted Date (Submitted_Date__v) |
Active Dossier Item Detail | Submitted Date | This lookup field shows the Actual Submission Date value of the related Submission record. |
Template (template__v) |
Active Dossier Item Detail | Template, Section | When blank, the source document’s Document Type determines which template section in which to add records. In this case, the Root Template must also be specified if you are intending to populate Active Dossier for any hierarchy that is not the default Common Technical Document (CTD) - Marketing hierarchy (Vault RIM UUID of f2d76f48-91bc-4a31-b254-3cdaaa81fc14). Otherwise, the Template field is required if the document type is not mapped in the Active Dossier Template or RIM Reference Model. |
Translation Document (translation_document__v) |
Active Dossier Item Detail | Translation Document |
Related Permissions for General Active Dossier Use
The sections below describe the minimum object permissions required for users to work within both the Active Dossier Viewer and Editor. All permissions are located in Admin > Users & Groups > Permission Sets.
Objects & Tabs
The permissions described below are located in the Objects or Tabs permission set tabs. For objects, you can also set these by clicking on the object and making selections under the Object Permissions heading.
Note: If Dynamic Access Control is enabled in your Vault for any of the below objects, a corresponding matching or custom sharing rule must be in place to ensure users can view and work with records.
| Object or Tab | Permission | Controls |
|---|---|---|
| Active Dossier Item, Active Dossier Item Detail, Active Dossier Structure |
Read, Create, Edit | Read: View Active Dossier records in the Viewer. Create, Edit: Ability to access the Add to Active Dossier dialog from the Editor and create Active Dossier records. Note: Create and Edit functions are also controlled by field permissions. See Object Fields below. |
| Application | Read | Ability to open the Editor from a Regulatory Objective, Submission, or Event record. |
Active Dossier Processing Issue (active_dossier_processing_issue__v) |
Read, Edit | Read: View Active Dossier Processing Issue records. Edit: Ability to edit Active Dossier Processing Issue records and mark them as Acknowledged. |
Active Dossier Processing (active_dossier_processing__v) |
Read | Read: View Active Dossier Processing records and view the hovercard within AD Viewer and on Event, Submission, and Regulatory Objective records to view the five most recent Active Dossier Processes. |
Active Dossier Processing Validation (active_dossier_processing_validation__v) |
Read, Edit | Read: View Active Dossier Processing Validation records. Edit: Ability to edit Active Dossier Processing Validation records in order to set them as Active or Inactive. |
Active Dossier Template (active_dossier_template__v) |
Read | Read: View Active Dossier records in the Viewer as well as utilize the Editor when there are multiple Active Dossier hierarchies in the Vault. Read access on the Root Template field is also required for proper access when there are multiple Active Dossier hierarchies. |
Active Substance (drug_substance__v) |
Read | Ability to open the drag and drop dialog from the Editor. |
| Activity | Read | Ability to open the Editor from an Event record. |
| Application | Read | Ability to open the Editor from a Regulatory Objective, Submission, or Event record. Read access to the Active Dossier Template field is required to properly load the Viewer and Editor when there are multiple Active Dossier hierarchies in the Vault. |
| Country | Read | Ability to filter and view Active Dossier records by Regulatory Objective in the Viewer and open the drag and drop dialog from the Editor. |
| Country Decision Detail | Read | Ability to view Active Dossier records in the Viewer and Editor. |
| Event | Read | Ability to open the Editor from an Event record. |
Inactive Ingredient (excipient__v) |
Read | Ability to open the drag and drop dialog from the Editor. |
| Manufacturer | Read | Ability to open the drag and drop dialog from the Editor. |
Product (drug_product__v) |
Read | Ability to open the drag and drop dialog from the Editor. |
Product Family (product__v) |
Read | Ability to view Active Dossier records in the Viewer and open the drag and drop dialog from the Editor. |
| Product Family Application | Read | Ability to filter and view Active Dossier records by Product Family then by Application in the Viewer and Editor. |
| Regulatory Objective | Read, Edit | Ability to open the Editor from a Regulatory Objective, Submission, or Event record. |
| Submission | Read, Edit | Ability to open the Editor from a Regulatory Objective, Submission, or Event record. |
| Submission Country | Read | Ability to filter and view Active Dossier records by Submission in the Viewer and Editor. |
| Tabs: Active Dossier | View | Ability to access the Viewer from the tab. |
Object Fields
The Active Dossier Item and Active Dossier Item Detail object fields listed below are minimally required to access and perform tasks within the Viewer and Editor.
The permissions described are located in the permission set tab Objects > [Object] > Object Field Permissions.
Note: Fields marked with an asterisk (*) are collectively the minimally-required fields to which users must have Edit access in order to create Active Dossier records in the Editor. If a user is missing Edit permission to any of them, the drag-and-drop action fails.
| Field | Permission | Controls |
|---|---|---|
| Active Dossier Item (Active Dossier Item Detail object only) | Read | View Active Dossier records in the Viewer. |
| Active Dossier Item | Read | View and filter by field |
| Active Dossier Item Detail | Read | View and filter by field |
| Active Dossier Status* (Active Dossier Item Detail object only) | Read, Edit | Read: View Active Dossier records in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Active Substance* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Active Substance Manufacturer* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Activity | Read, Edit | Read: View and filter by field, as well as view the field in the hovercard in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Application* | Read, Edit | Read: View and filter by column, as well as view the field in the hovercard in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Clinical Study | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Country (Active Dossier Item Detail object only) | Read | View Active Dossier records in the Viewer. |
| Country Code (Active Dossier Item Detail object only) | Read | View Active Dossier records in the Viewer. |
| Created By | Read | View and filter by field |
| Created Date | Read | View and filter by field |
| Document | Read | Read: View and filter by field, as well as view document details in the hovercard in the Viewer. |
| Event | Read, Edit | Read: View and filter by field, as well as view the field in the hovercard in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Hierarchy Path (Active Dossier Item, Active Dossier Item Detail, Active Dossier Structure objects) | Read | View Active Dossier records in the Viewer. |
| Inactive Ingredient* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Inactive Ingredient Manufacturer* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Indication | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Latest Change Comments | Edit | Enter optional comments when performing an edit or delete in the Active Dossier Editor |
| Metadata Search String (Active Dossier Item and Active Dossier Item Detail objects) | Read | View Active Dossier records in the Viewer. |
| Modified By | Read | View and filter by field |
| Modified Date | Read | View and filter by field |
| Nonclinical Study | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Product* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Product Family* | Read, Edit | Read: Ability to load the Viewer or Editor. Edit: The ability to Add to Active Dossier in the Editor. |
| Product Manufacturer* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Product Variant* | Read, Edit | Read: View and filter by field in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Region | Read | Ability to load the Viewer or Editor. |
| Reason for Change | Edit | Enter the required reason when performing an Edit, Move, or Delete action in the Active Dossier Editor. Not required for Confirm Pending or when editing the Comment field only |
| Regulatory Objective* | Read, Edit | Read: View and filter by field, as well as view the field in the hovercard in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Submission* | Read, Edit | Read: View and filter by field, as well as view the field in the hovercard in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
| Submissions Archive Document | Read | View document details in the hovercard in the Viewer. Users must also have corresponding permissions to view the document. |
| Translation Document | Read | View document details in the hovercard in the Viewer. Users must also have corresponding permissions to view the document. |
| Any applicable country field, for example Afghanistan (AF)* |
Read, Edit | Read: View and filter by country in the Viewer. Edit: The ability to Add to Active Dossier in the Editor. |
Object Actions
The permissions described below are located in the permission set tab Objects > [Object] > Object Action Permissions.
| Object | Action | Permission | Controls |
|---|---|---|---|
| Event | View Active Dossier | View, Execute | Ability to open the Viewer, filtered by the event or event’s relationships. |
| Event | Edit Active Dossier | View, Execute | Ability to open the Editor, filtered by the event or event’s relationships. |
| Submission | View Active Dossier | View, Execute | Ability to open the Viewer, filtered by the submission or submission’s relationships. |
| Submission | Edit Active Dossier | View, Execute | Ability to open the Editor, filtered by the submission or submission’s relationships. |
| Regulatory Objective | View Active Dossier | View, Execute | Ability to open the Viewer, filtered by the regulatory objective or regulatory objective’s relationships. |
| Regulatory Objective | Edit Active Dossier | View, Execute | Ability to open the Editor, filtered by the regulatory objective or regulatory objective’s relationships. |
Related Permissions for Registrations & Product Information Tracking
In addition to the permissions for general Active Dossier use, Registrations users should also be granted the below permissions in order to work with registration and product information tracking in the Active Dossier Viewer and Editor.
All permissions are located in Admin > Users & Groups > Permission Sets.
Objects
The permissions described below are located in the Objects permission set tab. You can also set these by clicking on the object and making selections under the Object Permissions heading.
Note: If Dynamic Access Control is enabled in your Vault for any of the below objects, a corresponding matching or custom sharing rule must be in place to ensure users can view and work with records.
| Object | Permission | Controls |
|---|---|---|
| AD Item Detail Registration | Read, Create, Delete | Read: Filtering by Registration in the Viewer and Editor, view access to the Registration column in the Active Dossier Item Detail page layout. Create: Create object records of this type. Delete: Delete object records of this type. Create or Delete: Inline and bulk editing in the Editor. |
| AD Item Detail Product Variant | Read, Create, Delete | Read: View access to the Product Variant column in the Active Dossier Item Detail page layout. Create: Create object records of this type. Delete: Delete object records of this type. Create or Delete: Inline and bulk editing in the Editor. |
| AD Item Detail Manufacturer | Read, Create, Delete | Read: View access to the Manufacturer column in the Active Dossier Item Detail page layout. Create: Create object records of this type. Delete: Delete object records of this type. Create or Delete: Inline and bulk editing in the Editor. |
| AD Item Detail Inactive Ingredient | Read, Create, Delete | Read: View access to the Inactive Ingredient column in the Active Dossier Item Detail page layout. Create: Create object records of this type. Delete: Delete object records of this type. Create or Delete: Inline and bulk editing in the Editor. |
| Registration: At least one object type | Read | Filtering by Registration in the Viewer and Editor, view access to the Registration column in the Active Dossier Item Detail page layout. |
Product Variant (product_detail__v): At least one object type |
Read | View access to the Product Variant column in the Active Dossier Item Detail page layout. |
| Manufacturer: At least one object type | Read | View access to the Manufacturer column in the Active Dossier Item Detail page layout. |
Inactive Ingredient (excipient__v): At least one object type |
Read | View access to the Inactive Ingredient column in the Active Dossier Item Detail page layout. |
Object Fields
The below Active Dossier Item Detail relationship object field permissions are required for product and information tracking, and are located in the permission set tab Objects > [Object] > Object Field Permissions.
| Object | Field | Permission | Controls |
|---|---|---|---|
| AD Item Detail Registration | Registration | Read | Filtering by Registration in the Viewer and Editor, view access to the Registration column in the Active Dossier Item Detail page layout. |
| AD Item Detail Product Variant | Product Variant | Read | View access to the Product Variant column in the Active Dossier Item Detail page layout. |
| AD Item Detail Manufacturer | Manufacturer | Read | View access to the Manufacturer column in the Active Dossier Item Detail page layout. |
| AD Item Detail Inactive Ingredient | Inactive Ingredient | Read | View access to the Inactive Ingredient column in the Active Dossier Item Detail page layout. |