The Active Dossier structure in RIM Submissions and RIM Submissions Archive Vaults helps sponsors maintain a list of current documents for a given product and market. Vault automatically populates the Active Dossier structure for a given submission, and calculates the current status for a regulatory objective and its related submissions based on source content in your Vault. In countries where submissions must be provided to the Health Authority in the local language, Vault also includes the translation source files in the Active Dossier structure. For Vaults which also have RIM Registrations licensed, Vault automatically populates the Active Dossier structure and calculates the current status when a Global Content Plan is dispatched.
You can use the Active Dossier Viewer to see content that is currently approved and the Active Dossier Editor to add new documents directly into the hierarchy. See Working with Active Dossiers for more information.
Note: This feature is only available on RIM Vaults. An Admin must configure Active Dossier in your Vault.
About the Active Dossier
Vault represents the Active Dossier structure in a common dossier format based on the specified Active Dossier Template. Sections within the template relate to source document types in Vault that are relevant for the Active Dossier. Vault automatically creates and populates the Active Dossier Structure based on changes to Submission and Regulatory Objective records.
Submissions Archive Source Document Detection
Vault automatically populates the Active Dossier with documents based on source content in your Vault. When you import a submission, Vault creates a Source References relationship between the source document and the Submissions Archive-type document when the file’s checksum matches. If an active Content Plan Item record in the submission content plan has a Published Output Location that corresponds to a file location in the current import, Vault uses the matched document as the source for the Submissions Archive-type document as well.
How Vault Creates & Updates the Active Dossier
When a Submission record enters the Health Authority Received state, Vault automatically creates the Active Dossier structure based on the source documents and document types of matched documents from Submission Content Plans. If a source document’s type corresponds to a document type defined in the Active Dossier Template, Vault includes the document in the Active Dossier.
Vault creates and updates records asynchronously.
From a Global Content Plan Dispatch
When creating or updating the Active Dossier from a Global Content Plan dispatch, Vault:
- Retrieves the relevant active Activities related to the Event with a Local Disposition value that is configured in one of the disposition categories on the Global Content Plan dispatch user action.
- Records are created per Activity, and are set with the Related Application, Regulatory Objective, and Related Submission values on the Activity
- Records are only created for Activities where the Global Content Plan was successfully dispatched to create a Submission Content Plan or comparison for the target submission. Currently, Vault does not create records when there is no target Submission, or the dispatch skips a target Activity if, for example, they are set with a Local Disposition of “No Action” or “Not Applicable”.
- For each relevant Activity, only considers documents matched to Global Content Plan Items or Content Plan sections that meet the following criteria:
- Document Set is blank, or the selected value matches the Document Set on the Activity.
- Lead Market Country and Region are blank, or the selected value matches the Impacted Market or the Impacted Market’s region on the Activity
- The matched document appears within a section that is within the dispatch scope.
- An active Content Plan Item does not have a “Delete” XML Operation value.
- If Copy Relationships is disabled for the dispatch, only documents within sections referencing Submission Product, Submission Active Substance, and Submission Inactive Ingredient relationships that exist on the target Submission’s relationships are considered.
- If Ignore Content Plan Template Constraints is disabled for the dispatch, only documents matched to records that are not excluded by template constraints on the target submission are included.
- Retrieves the relevant active Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship records from the Global Submission to create corresponding Active Dossier Structure records based on the Active Dossier Template
- Retrieves the source document based on the matched documents in each Content Plan Item in the Submission Content Plan. Matched documents must be version locked on the Content Plan Item to be considered.
- Creates the Active Dossier Item record based on the metadata from the Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship record referenced on the Content Plan where the document is used.
- For documents within Content Plan sections that do not have a Submission Product, Submission Active Substance, or Submission Inactive Ingredient defined, records are created based on matching values between the document’s Product Family and the Application’s associated Product Families.
- For Applications where the Enable Active Dossier Generation (
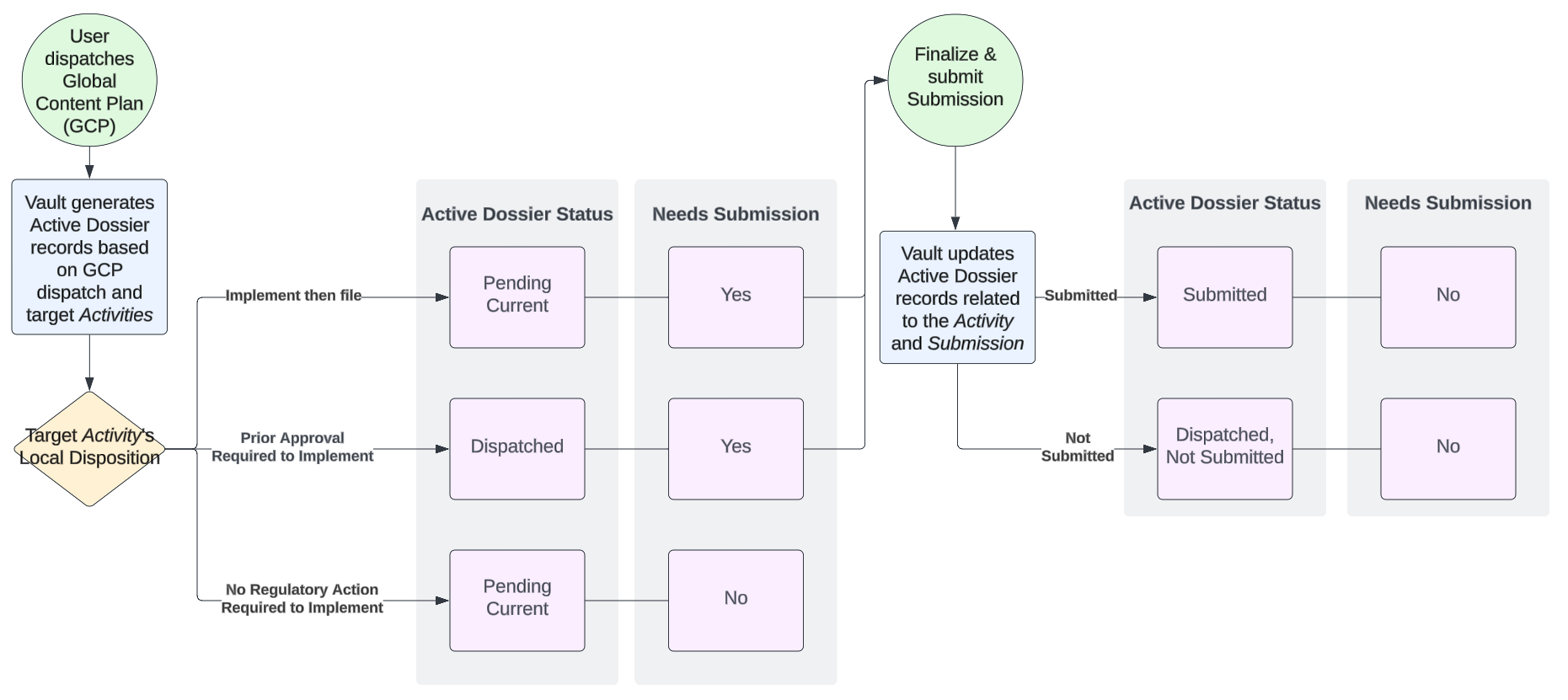
enable_active_dossier_generation__v) field is set to No, Active Dossier records are not created. - Records are created in either “Pending Current” or “Dispatched” status, depending on the Activity’s Local Disposition.
- For records created in “Pending Current”, Vault performs a calculation using the document version tree to set Active Dossier Item Details for older versions to Pending Superseded (if Current) or Replaced (if Pending Current or Dispatched). If there is a later version of the document to be proposed as Pending Current that is already Current or Pending Current, the Active Dossier status of the earlier version is set to Replaced instead of being set to Pending Current. The Originates From relationship allows you to relate the documents that the current document version should replace/supersede in the Active Dossier.
- Documents that have been dispatched but not submitted are set to Dispatched, Not Submitted.
- Records are automatically generated when using cross-template dispatches if the Populate Active Dossier checkbox on the GCP dispatch is enabled. Active Dossier records are created even for documents that are unmapped or later rejected in the comparison.
- The End Date field is defaulted with the current date on Active Dossier Item Details (ADIDs) that are moved to Pending Superseded/Deprecated or blank status as part of the current calculation.
- The Dispatch Date is defaulted with the current date on ADIDs created from a GCP dispatch.
- The Latest for Authoring and Latest for Authoring End Date fields are updated upon creation of new Active Dossier Item Details from a Global Content Plan Dispatch.
The diagram below provides a high-level illustration of how Vault sets Active Dossier Item Detail record fields according to the dispatched Activity’s Local Disposition field.
From Submissions Archive
When creating or updating the Active Dossier from Submissions Archive, Vault:
- Retrieves any active Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship records to create corresponding Active Dossier Structure records based on the Active Dossier Template
- Determines the ID and version of each document within the Submissions Archive dossier
- Retrieves the source document for each Submissions Archive-type document based on the Source References relationship
- Adds to the Active Dossier source documents whose metadata (Product, Product Variant, Manufacturer, Active Substance, Inactive Ingredient) match a Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship record.
- For eCTD submissions, the XML fields on the Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship record must also match the XML metadata for the leaf in Submissions Archive.
- Vault creates or updates the Active Dossier Item record using the metadata from the matching relationship record.
- For documents with only Product Family defined, Vault creates records based on the match with the Application’s associated Product Families.
- Populates or updates the Active Dossier Status field to Submitted, as well as setting the Document field with the Submissions Archive document, within any newly-created or updated Active Dossier Item and Active Dossier Item Detail records.
- Updates Needs Submission to “No” (false) if set to “Yes” (true) on updated Active Dossier Item Detail records.
- The Latest for Authoring and Latest for Authoring End Date fields are updated upon creation of new Active Dossier Item Details.
When the Disable Archive Population Path setting is configured, creating or updating the Active Dossier from Submissions Archive is disabled.
From a Submission Content Plan
When creating or updating the Active Dossier from a Submission Content Plan, Vault:
- Retrieves any active Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship records to create corresponding Active Dossier Structure records based on the Active Dossier Template
- Retrieves the source document based on the matched documents in each Content Plan Item in the Submission Content Plan. Matched documents must be version locked on the Content Plan Item to be considered.
- Vault ignores items that are inactive or have a “Delete” XML Operation.
- Creates or updates the Active Dossier Item record based on the metadata from the Submission Product, Submission Active Substance, or Submission Inactive Ingredient relationship record referenced on the Content Plan where the document is used.
- For documents within Content Plan sections that do not have a Submission Product, Submission Active Substance, or Submission Inactive Ingredient defined, records are created or updated based on matching values between the document’s Product Family and the Application’s associated Product Families.
- Populates or updates the Active Dossier Status field to Submitted, as well as setting the Content Plan and Content Plan Item fields, within any newly-created or updated Active Dossier Item and Active Dossier Item Detail records.
- Updates Needs Submission to “No” (false) if set to “Yes” (true) on updated Active Dossier Item Detail records.
- The Latest for Authoring and Latest for Authoring End Date fields are updated upon creation of new Active Dossier Item Details.
- When the Enable Active Dossier Global Tracking checkbox is configured on the Populate Active Dossier system action, Vault updates existing global Active Dossier records created from Global Content Plan dispatch from ‘Dispatched’ to ‘Submitted’ when the same document version is submitted locally, ignoring if the document was submitted under the same content plan context.
- To access the system action action to enable the checkbox, navigate to Admin > Configuration > Workflows, select the relevant Workflow (for example, “Generate AD records from Submissions”), then select the Populate the Active Dossier action.
When the Disable Archive Population Path setting is configured, Vault sets the Archive document fields if the document was published based on the Archive document fields linked in the Content Plan Item
For Registration & Product Information Tracking
When Active Dossier Registration Tracking is configured in a Registrations Vault and the Active Dossier is populated from a Global Content Plan dispatch or Submission Content Plan, Vault automatically creates Active Dossier Item Detail relationship records for Registrations, Product Variants, Manufacturers, and Inactive Ingredients based on matching fields between related Registrations, Active Dossier Item Details, and document metadata per the below logic.
First, Vault determines in-scope Registrations according to a record’s:
- Lifecycle state, where the record must be in a state which maps to a configured state type.
- Referenced Regulatory Objective (via the Registration Regulatory Objective relationship), or its referenced Application. Vault uses the referenced Application when the Active Dossier Item Detail record:
- Has a blank Regulatory Objective field, or
- The record does not have any Registration Regulatory Objective relationships.
- Referenced Country which matches an Active Dossier Item Detail. Depending on your Vault’s configuration and the procedure type, in-scope Country records can be further filtered based on Procedure Type Country Constraints.
- Relationships, where the Registration must be linked to at least one Registered Product and/or Registered Active Substance that is in a configured state type and matches the Product or Active Substance on the Active Dossier Item Detail.
Then, Vault creates the Active Dossier Item Detail relationship records accordingly, depending on your configuration.
- Registrations are tracked via AD Item Detail Registration records. Vault creates these records according to the same logic it uses to determine the overall in-scope Registration records.
- Manufacturers are tracked via AD Item Detail Manufacturer records.
- Vault creates these records for each Manufacturer populated on Registered Product, Registered Active Substance or Registered Inactive Substance, and Registration records in the configured state type. Vault only considers records set with a Manufacturing Site Role value that is not excluded from Active Dossier.
- The above records must also match the values set on the Active Dossier Item Detail record and the related document. A blank field or record where the Special Record Classification field is set matches with any value.
- Product Variants are tracked via AD Item Detail Product Variant records.
- Vault creates these records for each Product Variant populated on Registered Product and Registration records in the configured state type.
- The above records must also match the values set on the Active Dossier Item Detail record and the related document. A blank field or record where the Special Record Classification field is set matches with any value.
- Inactive Ingredients are tracked via AD Item Detail Inactive Ingredient records.
- Vault creates these records for each Inactive Ingredient populated on Registered Inactive Ingredient and Registration records in the configured state type.
- The above records must also be referenced by the Active Dossier Item Detail record and the related document. A blank field or record where the Special Record Classification field is set matches with any value.
About Translation Documents
In countries where submissions are provided to the Health Authority in the local language, Vault identifies the translation source document for inclusion within the Active Dossier. Vault includes the document in the English language as the Active Dossier Item record, and captures the non-English language document as an Active Dossier Item Detail record that you can reference.
If the Language document field is populated with a value that does not include English, Vault uses the Translation document relationship type to retrieve the English language source document. Vault creates Active Dossier Item and Active Dossier Item Detail records for each source document in the Translation document relationship of the non-English document.
How Vault Sets Latest for Authoring
Vault automatically sets Latest for Authoring (LFA) when a new Active Dossier Item Detail (ADID) is created from population processes, drag and drop, or Active Dossier Loader. When setting the Latest for Authoring field, Vault pulls the ADIDs for any earlier and later versions of that document to determine whether the document version that the ADID is being created for is the latest version in its document hierarchy. This can be by:
- The document version of the document (for example, v3.0 is later than v2.0 of the same document ID)
- Using the Originates From document relationship. If a document B originates from document A, then document B is considered to be later than document A.
If Vault finds an ADID for a later version of the document the new ADID is being created for, then the new ADID will be set with LFA = No.
If Vault does not find a later version, it sets the document’s LFA to Yes. Vault looks at the ADIDs representing previous versions of the document version and sets the previous version that it finds that has LFA value of “Yes” to “No”. For example, if an ADID is created for v3.0,Vault sets the ADID with v2.0 that was previously LFA = Yes to LFA = No. In this case, Vault also sets the Latest for Authoring End Date with the date that the value was changed from Yes to No.
Vault calculates the LFA within the scope of the same Application-, Country-, and Product-related metadata.
Special Handling for Published Report Level Content Plan Documents
During Active Dossier generation, if the document is the published output from a Report Level Content Plan (Published by RLCP field is set to Yes), Vault retrieves the source document(s) in the Source References relationship of the published report document to generate the Active Dossier Item and Active Dossier Item Detail records.
The Published Report Document field is populated with the published RLCP document version. The Active Dossier records are created based on the source document’s document type and not the published report document’s document type. If there are multiple documents in the Published Report Document’s Source References relationship, Active Dossier records are created for each document version reference in the relationship.