European Union regulations require medical device manufacturers to submit Unique Device Identification (UDI) data for medical devices to the European Databank on Medical Devices (EUDAMED) in XML format.
The UDI Submission Viewer presents these complex XML files in a user-friendly format, allowing your organization to:
- Review Vault-identified validation errors.
- Flag problematic or missing content.
- Download a report which includes validation error details, user comments, and suggestions for how to correct source data.
- Change the states of the related records and XML file to reflect the review outcome.
- Submit UDI data to EUDAMED directly from a Registrations Vault, when configured.
Note: This feature is only available on RIM Registrations Vaults and must be configured by an Admin.
Launching the Viewer
When you generate UDI data from a Registration, Vault creates a UDI Submission record, relates it to the Registration record, and sends a notification confirming the results of the operation.
When the UDI Submission Viewer is enabled, Vault also:
- Sets the UDI Submission object record state to indicate whether the submission is Ready to Review or that it Failed to Generate.
- Includes a link to these new records in the results notification.
To launch the viewer:
- Open the relevant UDI Submission record from the notification, or otherwise navigate to a UDI Submission record in the Ready to Review state.
- Select Review UDI Submission from the record’s Actions menu.
Navigating the Viewer
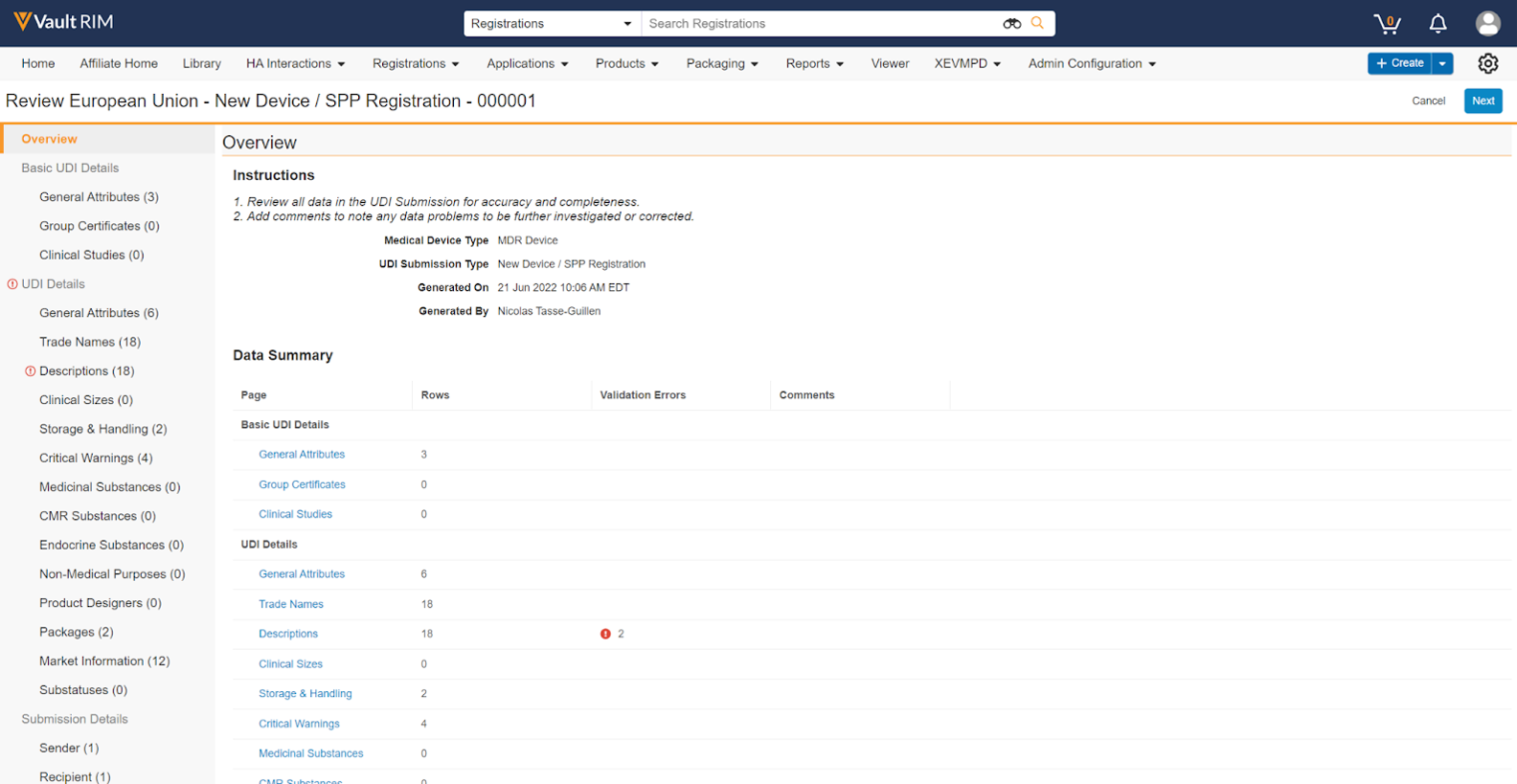
Overview Page
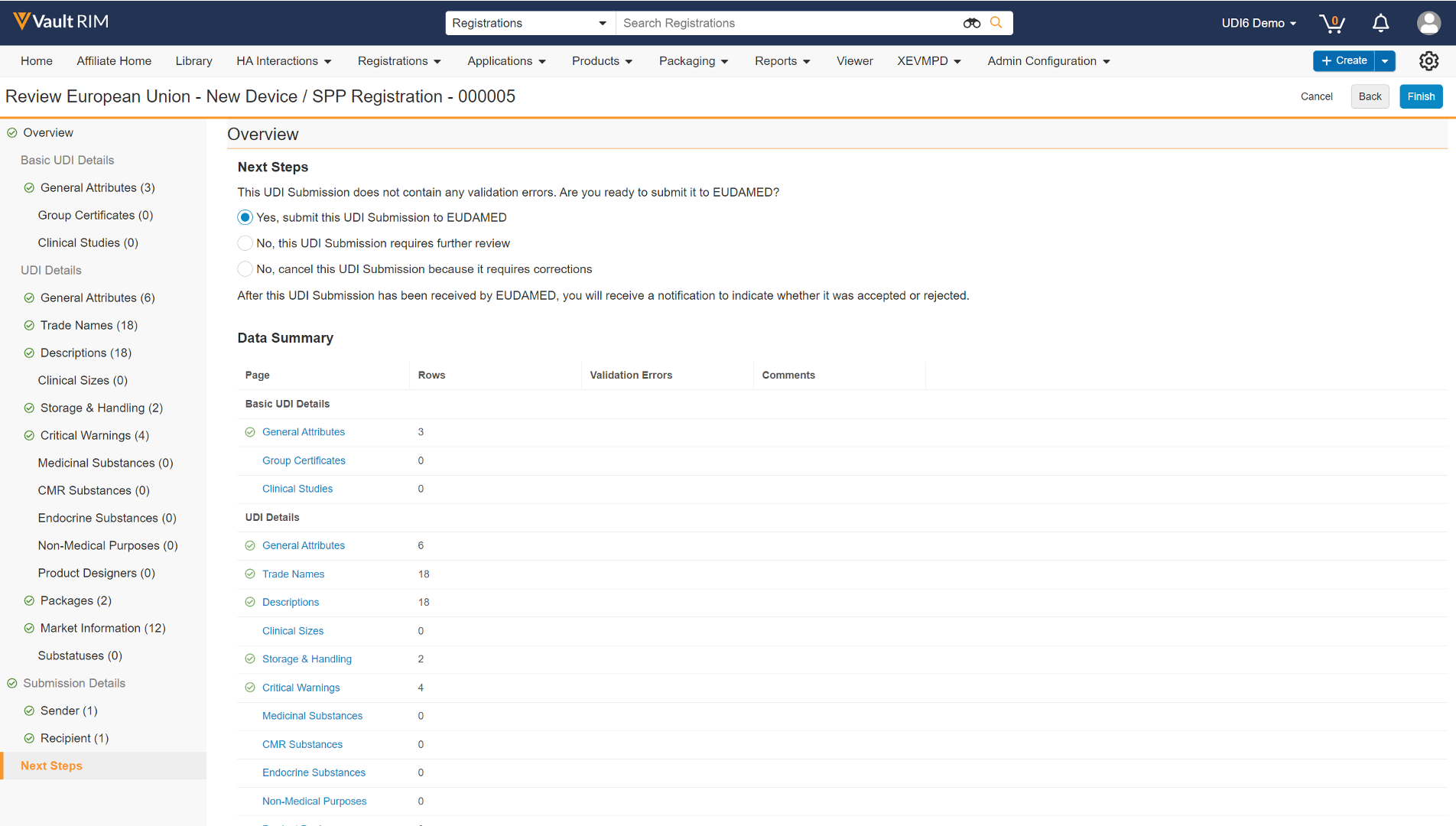
When you launch the UDI Submission Viewer, Vault first displays an Overview page, with Instructions and relevant metadata. The page also includes a Data Summary of all UDI Group, UDI, and Submission details, with the numbers of Rows and Validation Errors detected for each.
You can review each data category (for example, General Attributes) in any order using the links in the left-hand navigation panel, or click the Next and Back buttons to traverse each data category page in order.
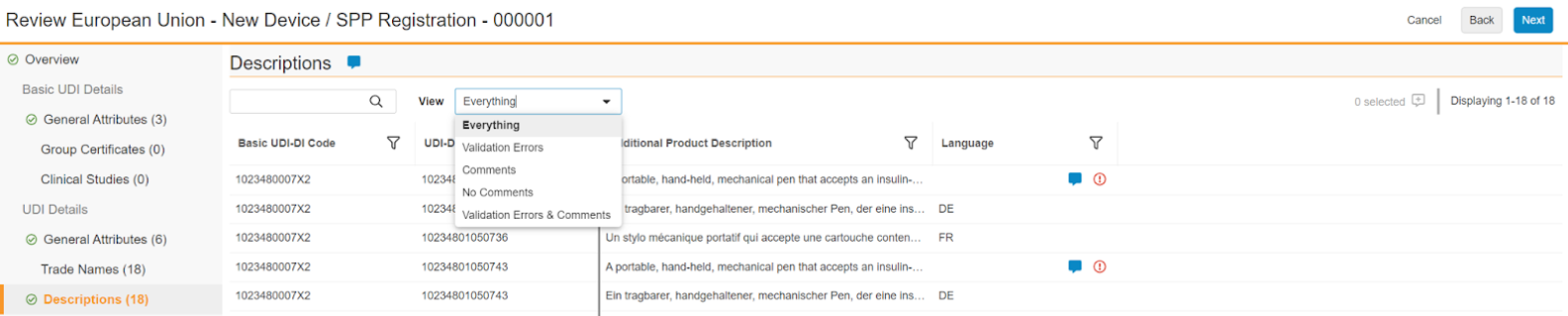
Data Category Pages
Each data category page is a grid of columns applicable to the Medical Device Type of the UDI Submission. Depending upon the data displayed for the category, you can review validation errors, add comments where corrections may be required, and filter and search.
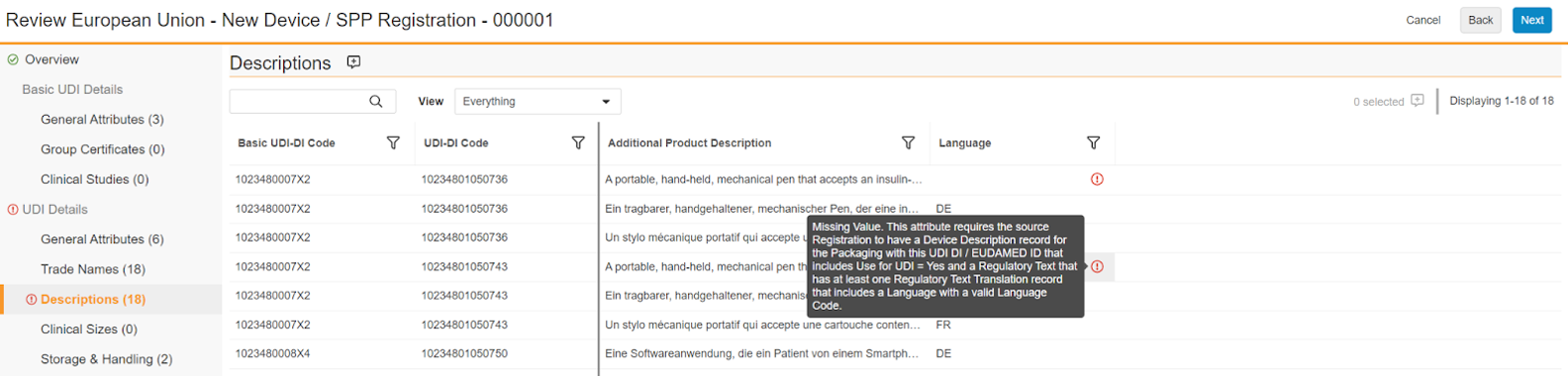
Reviewing Validation Errors
In the columns where there are validation errors, you can hover over the red exclamation circle icon to display Vault’s suggestions for how to fix the source data. These suggestions are also provided within the summary report Vault generates at the end of the review process.
Commenting
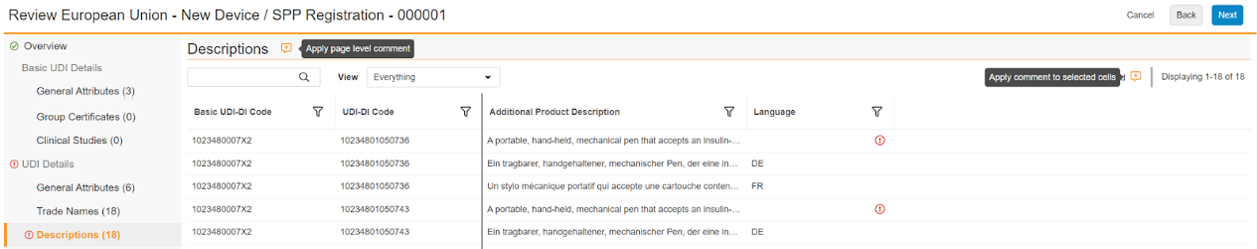
You can use the viewer’s commenting feature to annotate validation errors and other displayed data that is technically valid but requires correction, such as a misspelling.
To add a comment for the entire page, click the comment icon to the right of the data category heading. To add a comment for one or more cells, click the comment icon to the left of the record count for the page. Then, enter text in the Comment dialog and click Save.
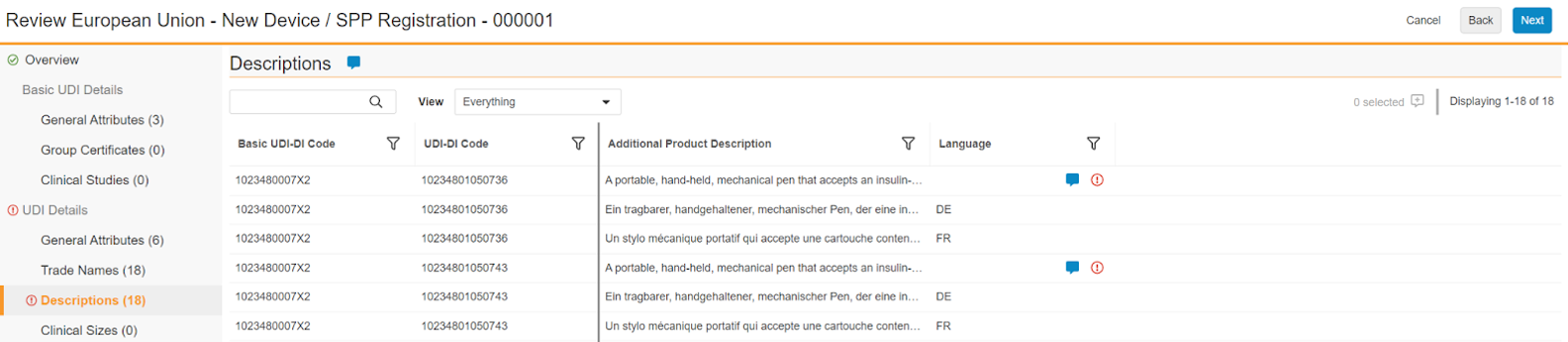
Pages and cells with saved comments appear with a solid blue comment icon:
Searching, Filtering, and Monitoring Review Progress
As your review progresses, Vault updates the left-hand navigation panel with a green checkmark to indicate the page has been visited.
Additionally, throughout your review, you can perform a text search across all rows and all columns, and filter by each visible column.
When applicable, you can make a selection in the View dropdown to show Everything, rows with one or more Validation Errors or Comments only, No Comments, or all rows with Validation Errors & Comments.
Next Steps Page
When your review is complete, Vault displays the Next Steps page with options on how to proceed, based upon whether the XML file contains validation errors.
The option to submit directly to EUDAMED appears when the submission does not contain any validation errors, and when your Vault is configured for machine-to-machine submission. See Direct Submissions from Vault for additional steps.
If your Vault’s viewer configuration does not include submission capability, in most cases, when you click Finish, Vault sends a report summarizing the review and updates the lifecycle states of related objects and documents accordingly.
See the following sections for more information:
- Pre-Submission Lifecycle State Updates
- Manual Uploads to EUDAMED
- About the UDI Submission Review Report
Pre-Submission Lifecycle State Updates
When you select a review verdict other than Requires further review, Vault updates the below components’ lifecycle states:
- UDI Submission object record
- Registered Device Identifier object records for the identifiers included in the submission
- UDI Submission XML document in the Library
The table below describes Vault behavior based upon review outcome. Object lifecycle state labels may differ in your Vault, depending upon your Admin’s configuration.
| Review Outcome | Object Lifecycle State | Document Lifecycle State |
|---|---|---|
| Requires further review | N/A – no change | N/A – no change |
| Cancel | Canceled | Canceled |
| Ready to submit to EUDAMED | Ready to Submit | Ready to Submit |
| Submit to EUDAMED (if configured) |
Sending to Health Authority | Sending to HA |
Submitting to EUDAMED
The UDI Submission Viewer allows you to submit UDI data to EUDAMED directly from your Registrations Vault. When configured by an Admin and the submission does not contain any validation errors, the Next Steps page includes the submission option.
Otherwise, see Manual Uploads to EUDAMED.
Direct Submissions from Vault
To submit UDI data to EUDAMED from the viewer:
- Launch the viewer and proceed through each Data Category page until all validation errors are cleared.
- Select Yes, submit this UDI Submission to EUDAMED, then click Finish.
In some cases, a UDI submission may fail to send or Vault may not receive a response from EUDAMED (for example, if EUDAMED is down for maintenance). If this occurs, you can attempt to resubmit the data from a UDI Submission record:
- Navigate to a UDI Submission record in the Ready to Submit or Sending to Health Authority state.
- Select Resubmit UDI Submission from the record’s Actions menu.
Vault transmits the submission to EUDAMED and sends you a notification with a link to the UDI Submission Review Report.
Depending on the submission outcome, Vault may also:
- Update the UDI Submission XML document’s lifecycle state.
- Update the UDI Submission and Registered Device Identifier object lifecycle states.
- Add EUDAMED acknowledgements as Attachments to the UDI Submission record.
- Add UDI Submission Error records to the UDI Submission for each data category with errors.
| Submission Outcome | Vault Action | Lifecycle State Change |
|---|---|---|
| Failed to send | Notification | Ready to Submit |
| No response | Notification | Ready to Submit |
| Unexpected or partial response | * Notification * Attachment on UDI Submission record |
N/A – no change |
| Rejected | * Notification * Attachment on UDI Submission record * UDI Submission Error records created for each data category related to UDI Submission record |
Rejected by HA |
| Accepted | * Notification * Attachment on UDI Submission record |
Accepted by HA |
Manual Uploads to EUDAMED
Submitting UDI data directly from Vault to EUDAMED is recommended, but it is possible to download an XML file generated by Vault to manually upload it to EUDAMED. Once a submission has been set to the Ready to Submit state, you can download the Submission Data File, which is an XML document in the Vault Library linked to the UDI Submission record.
Next, someone with appropriate permissions must log into EUDAMED and upload the XML file by navigating to Transmission > Upload > New Upload within EUDAMED. The service selected in EUDAMED must match the UDI Submission Type in Vault.
Once a submission has been accepted or rejected by EUDAMED, you must mark the UDI Submission and its related records accordingly before you can generate another submission for the same Registration.
Similar to the viewer’s automatic state change capabilities, when you select UDI Submission Accepted by HA or UDI Submission Rejected by HA from the Actions menu of a UDI Submission record, Vault updates the UDI Submission object record, Registered Device Identifier object records, and UDI Submission XML document lifecycle states accordingly.
Once Vault completes the state changes, you’ll receive a notification with the update details.
If the submission was rejected by EUDAMED, you will need to evaluate the errors in the EUDAMED Response file and fix your source data before generating another submission.
About the UDI Submission Review Report
After completing a review, Vault sends you a CSV file summarizing any validation errors encountered and comments applied during the review. The file also includes suggestions for correcting validation errors.
Vault also includes the report in notifications each time you submit to EUDAMED from the viewer.