The United Kingdom (UK) formally withdrew from the European Union (EU) on January 31, 2020. The transition period began on February 1, 2020 and ended on December 31, 2020. As of January 1, 2021, Great Britain will be considered a third country to the EU. This page outlines implications of Brexit for customers using RIM Vaults, including some actions customers can take in their Vaults to continue licensing or to license new products in either the UK or EU.
Note: Although the Withdrawal Agreement has been finalized, the MHRA continues to update their guidance. Check with the EMA and MHRA for the latest information.
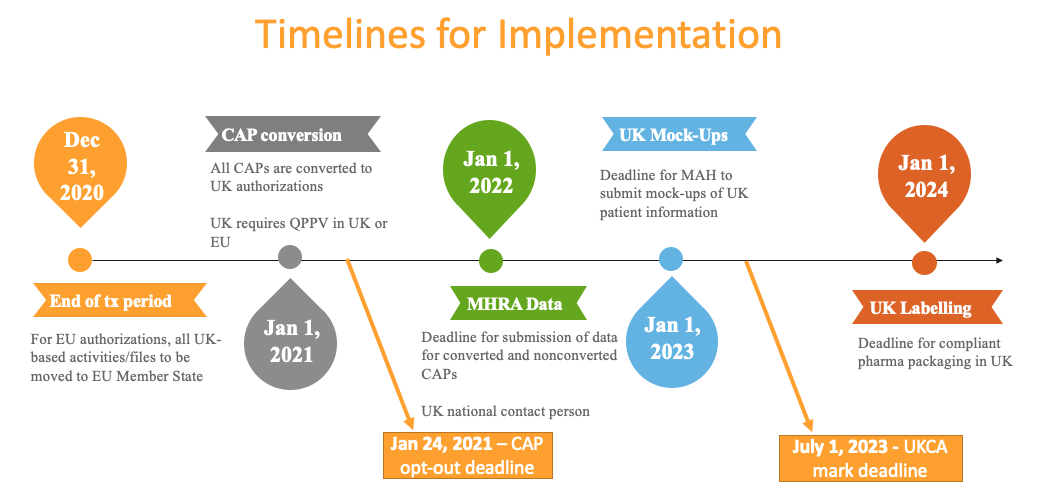
Timelines
Different timelines apply depending on whether the product is a pharmaceutical or a device, and whether or not the product is being converted into a GB MA (Marketing Authorization). This timeline provides an overview of required deadlines:
Impact for Pharmaceutical Products
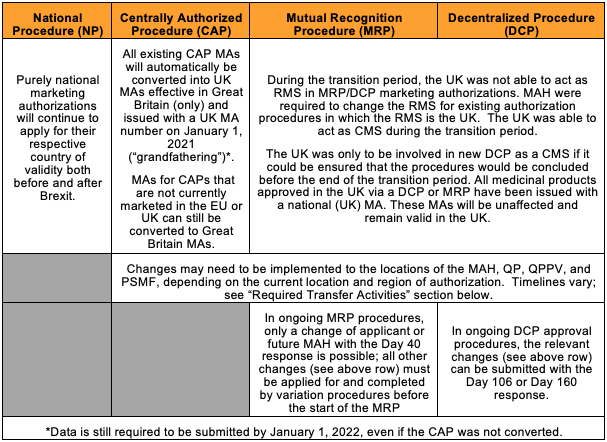
The impact of Brexit varies depending on the procedure type applied to the pharmaceutical product. Existing Centrally Authorized Procedure (CAP) products were converted into GB MAs automatically on January 1, 2021. Products authorized through a Decentralized Procedure (DCP) and Mutual Recognition Procedure (MRP) have been issued with GB national MAs and will be unaffected.
For products authorized by the centralized procedure, the MHRA requires a baseline submission before January 1, 2022, and will issue one or more Product License numbers based on existing UK practices for determining the number of separate national licenses needed across a product range. Generally, fewer GB MA numbers will be required in comparison to EC authorizations because all pack sizes for a presentation will be covered by a single MA number. Marketing Authorization Holders (MAHs) could opt-out of the conversion process for all or some of their CAPs by notifying the MHRA in writing by close of business on January 21, 2021. If the CAP was not grandfathered into a GB license, the MHRA still requires a baseline submission before January 1, 2022, for Northern Ireland.
This table provides a high-level summary of impact to existing EU market authorizations, as well as actions MAHs need to take:
Required Transfer Activities
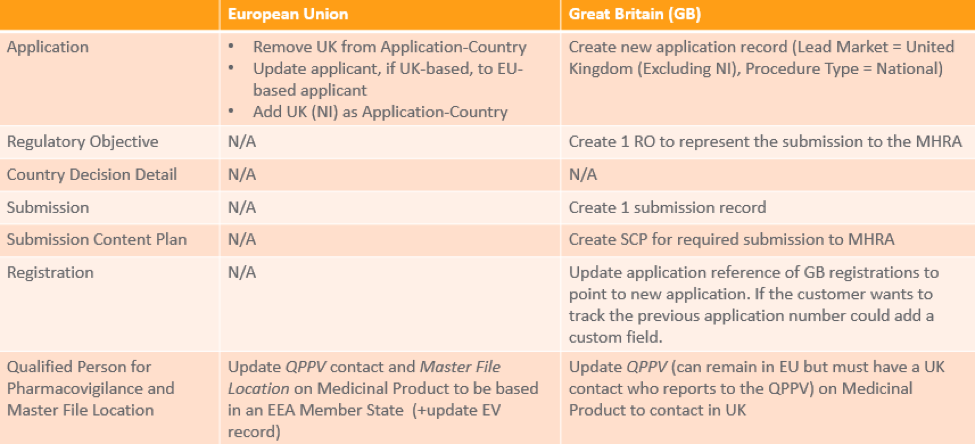
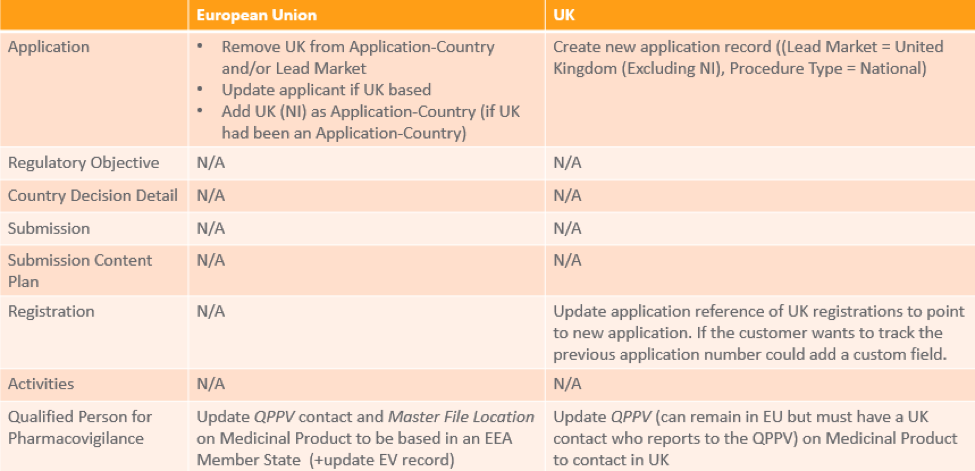
To continue marketing products and ensure that authorized products can remain on the market in the EU or the UK, the MAH needs to take certain steps at varying points. This table provides a summary of necessary activities in the EU or the UK:
Existing CAP Authorizations in Vault
This table provides information about managing existing CAPs in Vault, depending on where the product is marketed:
Existing MRP & DCP Authorizations in Vault
This table provides information about managing existing MRP or DCP authorizations in Vault, depending on where the product is marketed:
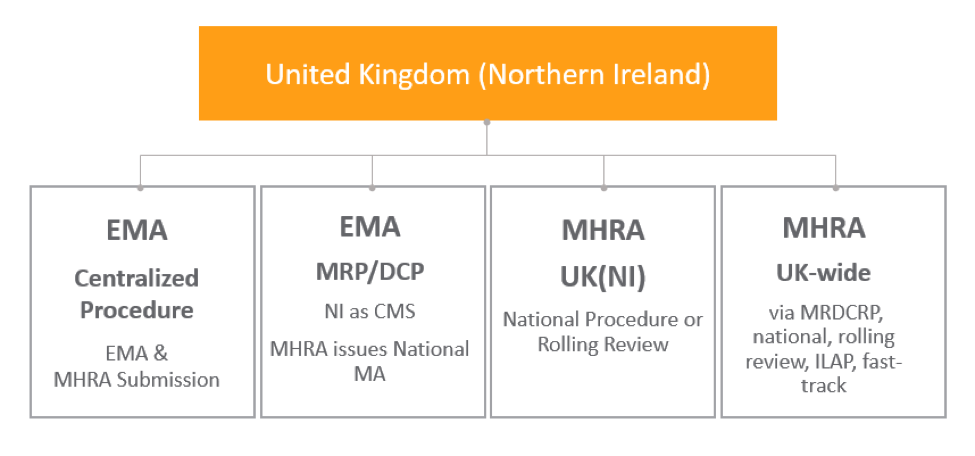
Northern Ireland
The Northern Ireland protocol was established to avoid a hard border between Northern Ireland and Ireland, which is an EU country. This protocol ensures that Northern Ireland will follow EU legislations and will have equal market access with the Republic of Ireland, despite not being a part of the EU.
For existing CAPs, if the product is marketed in Great Britain but not in Northern Ireland, the MAH should send an updated marketing status report to the EMA to alert them of the marketing cessation for Northern Ireland. If the CAP is marketed in Northern Ireland, no change in marketing status is required. New CAPs will continue to be valid in Northern Ireland, although a submission to the MHRA is also required.
This table shows how organizations can obtain Northern Ireland authorizations through MHRA or EMA procedures:
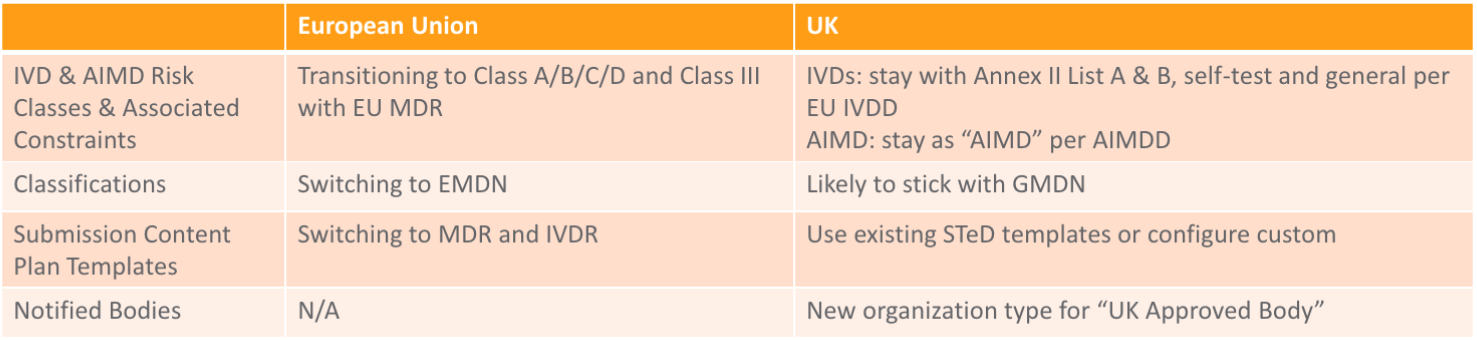
Impact for Medical Devices
Device manufacturers should have transferred their CE certificates to an EU Notified Body and obtained an EU-based Authorized Representative to ensure continued marketing after December 31, 2020.
Medical Devices in Vault
This table provides information about managing device applications in Vault, depending on where the product is marketed:
CE Marking & Registration
The MHRA will recognize devices bearing the CE Mark until June 30, 2023, including EU-based CE certificates (both AIMDD/MDD/IVDD and MDR/IVDR). A new UKCA Mark will be available as of January 01, 2021, and will be mandatory for devices in the UK as of July 01, 2023. Starting on January 1, 2021, all medical devices and IVDs placed on the UK market will need to be registered with the MHRA, although a grace period will apply.
UKNI Marking
Devices placed on the market in Northern Ireland will continue to require the CE Mark and will need to meet EU rules. Starting on January 1, 2021, there are two options for marketing a device in Northern Ireland:
- CE Mark with EU Notified Body
- CE Mark with UK Notified Body + UKNI Mark
For more information about the acceptable conformity marks and required registration, see the latest guidance from the MHRA.
UK Responsible Person
Foreign manufacturers will need to establish a UK Responsible Person who will be responsible for registering the device with the MHRA. The UK Responsible Person must be in place as of January 1, 2021, regardless of the grace period for registering a device with the MHRA.
21R1 RIM Changes & Configuration Updates for Brexit
Any new RIM implementations after the 21R1 release automatically include the following updates to account for Brexit:
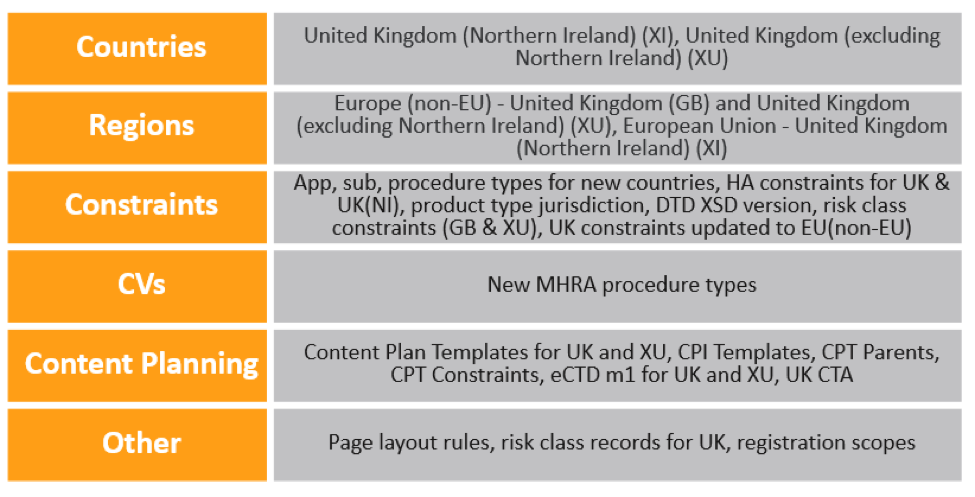
In existing Vaults, Admins must make the following configuration updates for Brexit changes:
- On the United Kingdom Country record, update the Region to Europe (Non-EU).
- Update the Region record called European Union to add the following Countries:
|
Country Name |
Region |
Country Code |
|
United Kingdom (Northern Ireland) |
European Union |
XI |
|
United Kingdom (excluding Northern Ireland) |
Europe (Non-EU) |
XU |
- Add Constraint records to include the relevant Application Types, Submission Types, Submission Subtypes, and Health Authorities for the newly-created countries.
- Update or add Constraint records of type Product Type Jurisdiction for the UK, XI, and XU country codes.
- Add Constraint records of type DTD XSD Version and constraints for UK-specific procedure types.
- Copy the EU Module 1 in existing submission content plans to add Module 1 for the GB and XU country codes. This recommendation may change depending on future MHRA guidance.
Note that the Create Registrations wizard will be updated to include United Kingdom (Northern Ireland) in a future release. Until then, users can add this value manually in the wizard. A future release will also include a new Organization Type of UK Approved Body for medical devices.
21R3 RIM Changes for Brexit
In 21R3 (21R2.3), the bulk registrations creation wizard in RIM Registrations Vaults automatically includes United Kingdom (Northern Ireland) (Country Code = XI) for applications where the Procedure Type is Centralised Procedure. Until 21R3, General Release Vault users can add this value manually in the wizard.